Abstract
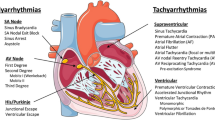
Aconitine (ACO), a main active ingredient of Aconitum, is well-known for its cardiotoxicity. However, the mechanisms of toxic action of ACO remain unclear. In the current study, we investigated the cardiac effects of ACO and mesaconitine (MACO), a structurally related analog of ACO identified in Aconitum with undocumented cardiotoxicity in guinea pigs. We showed that intravenous administration of ACO or MACO (25 μg/kg) to guinea pigs caused various types of arrhythmias in electrocardiogram (ECG) recording, including ventricular premature beats (VPB), atrioventricular blockade (AVB), ventricular tachycardia (VT), and ventricular fibrillation (VF). MACO displayed more potent arrhythmogenic effect than ACO. We conducted whole-cell patch-clamp recording in isolated guinea pig ventricular myocytes, and observed that treatment with ACO (0.3, 3 μM) or MACO (0.1, 0.3 μM) depolarized the resting membrane potential (RMP) and reduced the action potential amplitude (APA) and durations (APDs) in a concentration-dependent manner. The ACO- and MACO-induced AP remodeling was largely abolished by an INa blocker tetrodotoxin (2 μM) and partly abolished by a specific Na+/K+ pump (NKP) blocker ouabain (0.1 μM). Furthermore, we observed that treatment with ACO or MACO attenuated NKP current (INa/K) and increased peak INa by accelerating the sodium channel activation with the EC50 of 8.36 ± 1.89 and 1.33 ± 0.16 μM, respectively. Incubation of ventricular myocytes with ACO or MACO concentration-dependently increased intracellular Na+ and Ca2+ concentrations. In conclusion, the current study demonstrates strong arrhythmogenic effects of ACO and MACO resulted from increasing the peak INa via accelerating sodium channel activation and inhibiting the INa/K. These results may help to improve our understanding of cardiotoxic mechanisms of ACO and MACO, and identify potential novel therapeutic targets for Aconitum poisoning.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Singhuber J, Zhu M, Prinz S, Kopp B. Aconitum in traditional Chinese medicine: a valuable drug or an unpredictable risk? J Ethnopharmacol. 2009;126:18–30.
Lin MW, Wang YJ, Liu SI, Lin AA, Lo YC, Wu SN. Characterization of aconitine-induced block of delayed rectifier K+ current in differentiated NG108-15 neuronal cells. Neuropharmacology. 2008;54:912–23.
Chan TY. Aconite poisoning. Clin Toxicol. 2009;47:279–85.
Bartosova L, Novak F, Bebarova M, Frydrych M, Brunclik V, Opatrilova R, et al. Antiarrhythmic effect of newly synthesized compound 44Bu on model of aconitine-induced arrhythmia—compared to lidocaine. Eur J Pharmacol. 2007;575:127–33.
Amran MS, Hashimoto K, Homma N. Effects of sodium-calcium exchange inhibitors, KB-R7943 and SEA0400, on aconitine-induced arrhythmias in guinea pigs in vivo, in vitro, and in computer simulation studies. J Pharmacol Exp Ther. 2004;310:83–9.
Li Y, Tu D, Xiao H, Du Y, Zou A, Liao Y, et al. Aconitine blocks HERG and Kv1.5 potassium channels. J Ethnopharmacol. 2010;131:187–95.
Wang YJ, Chen BS, Lin MW, Lin AA, Peng H, Sung RJ, et al. Time-dependent block of ultrarapid-delayed rectifier K+ currents by aconitine, a potent cardiotoxin, in heart-derived H9c2 myoblasts and in neonatal rat ventricular myocytes. Toxicol Sci. 2008;106:454–63.
Wright SN. Comparison of aconitine-modified human heart (hH1) and rat skeletal (mu1) muscle Na+ channels: an important role for external Na+ ions. J Physiol. 2002;538:759–71.
Sun GB, Sun H, Meng XB, Hu J, Zhang Q, Liu B, et al. Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats. Toxicol Appl Pharmacol. 2014;279:8–22.
Wu J, Wang X, Chung YY, Koh CH, Liu Z, Guo H, et al. L-type calcium channel inhibition contributes to the proarrhythmic effects of aconitine in human cardiomyocytes. PLoS One. 2017;12:e0168435.
Zhou YH, Piao XM, Liu X, Liang HH, Wang LM, Xiong XH, et al. Arrhythmogenesis toxicity of aconitine is related to intracellular Ca2+ signals. Int J Med Sci. 2013;10:1242–9.
Britton OJ, Bueno-Orovio A, Virag L, Varro A, Rodriguez B. The electrogenic Na+/K+ Pump is a key determinant of repolarization abnormality susceptibility in human ventricular cardiomyocytes: a population-based simulation study. Front Physiol. 2017;8:278.
Guo D, Jenkinson S. Simultaneous assessment of compound activity on cardiac Nav1.5 peak and late currents in an automated patch clamp platform. J Pharmacol Toxicol Methods. 2018;99:106575.
Mitamura M, Horie S, Sakaguchi M, Someya A, Tsuchiya S, Van de Voorde J, et al. Mesaconitine-induced relaxation in rat aorta: involvement of Ca2+ influx and nitric-oxide synthase in the endothelium. Eur J Pharmacol. 2002;436:217–25.
Ogura J, Mitamura M, Someya A, Shimamura K, Takayama H, Aimi N, et al. Mesaconitine-induced relaxation in rat aorta: role of Na+/Ca2+ exchangers in endothelial cells. Eur J Pharmacol. 2004;483:139–46.
Liu QH, Li XL, Xu YW, Lin YY, Cao JM, Wu BW. A novel discovery of IK1 channel agonist: zacopride selectively enhances IK1 current and suppresses triggered arrhythmias in the rat. J Cardiovasc Pharmacol. 2012;59:37–48.
Wang YH, Shi CX, Dong F, Sheng JW, Xu YF. Inhibition of the rapid component of the delayed rectifier potassium current in ventricular myocytes by angiotensin II via the AT1 receptor. Br J Pharmacol. 2008;154:429–39.
Park MH, Park WS, Jo SH. Acute alteration of cardiac ECG, action potential, IKr and the human ether-a-go-go-related gene (hERG) K+ channel by PCB 126 and PCB 77. Toxicol Appl Pharmacol. 2012;262:60–9.
Wang C, Hennessey JA, Kirkton RD, Wang C, Graham V, Puranam RS, et al. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circ Res. 2011;109:775–82.
Yuill KH, Convery MK, Dooley PC, Doggrell SA, Hancox JC. Effects of BDF 9198 on action potentials and ionic currents from guinea-pig isolated ventricular myocytes. Br J Pharmacol. 2000;130:1753–66.
Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912.
Wang X, Tang H, Wei EQ, Wang Z, Yang J, Yang R, et al. Conditional knockout of Fgf13 in murine hearts increases arrhythmia susceptibility and reveals novel ion channel modulatory roles. J Mol Cell Cardiol. 2017;104:63–74.
Hamada K, Matsuura H, Sanada M, Toyoda F, Omatsu-Kanbe M, Kashiwagi A, et al. Properties of the Na+/K+ pump current in small neurons from adult rat dorsal root ganglia. Br J Pharmacol. 2003;138:1517–27.
Lee SY, Lee CO. Inhibition of Na+-K+ pump and L-type Ca2+ channel by glibenclamide in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 2005;312:61–8.
Wang Y, Gao J, Mathias RT, Cohen IS, Sun X, Baldo GJ. alpha-Adrenergic effects on Na+-K+ pump current in guinea-pig ventricular myocytes. J Physiol. 1998;509:117–28.
Li C, Zou R, Zhang H, Wang Y, Qiu B, Qiu S, et al. Upregulation of phosphoinositide 3-kinase prevents sunitinib-induced cardiotoxicity in vitro and in vivo. Arch Toxicol. 2019;93:1697–712.
Jakob H, Nawrath H. Tetrodotoxin slightly shortens action potential duration in ventricular but not in atrial heart muscle. Experientia. 1988;44:16–7.
Antzelevitch C, Nesterenko V, Shryock JC, Rajamani S, Song Y, Belardinelli L. The role of late INa in development of cardiac arrhythmias. Handb Exp Pharmacol. 2014;221:137–68.
Glitsch HG. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol Rev. 2001;81:1791–826.
Levi AJ, Dalton GR, Hancox JC, Mitcheson JS, Issberner J, Bates JA, et al. Role of intracellular sodium overload in the genesis of cardiac arrhythmias. J Cardiovasc Electrophysiol. 1997;8:700–21.
Gadsby DC, Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989;94:511–37.
Lewalle A, Niederer SA, Smith NP. Species-dependent adaptation of the cardiac Na+/K+ pump kinetics to the intracellular Na+ concentration. J Physiol. 2014;592:5355–71.
Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–9.
Zan K, Wangjie CR, Lu J, Guo LN, Zheng J, Ma SC. Content determination of four diester diterpenoid alkaloids in leaves of Aconitum kusnezoffii by HPLC. Zhongguo Zhong Yao Za Zhi. 2018;43:766–71.
Kiyosue T, Arita M. Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. Circ Res. 1989;64:389–97.
Song Y, Belardinelli L. Basal late sodium current is a significant contributor to the duration of action potential of guinea pig ventricular myocytes. Physiol Rep. 2017;5:e13295.
Ono T, Hayashida M, Tezuka A, Hayakawa H, Ohno Y. Antagonistic effects of tetrodotoxin on aconitine-induced cardiac toxicity. J Nippon Med Sch. 2013;80:350–61.
Wu Y, Wang L, Ma J, Song Y, Zhang P, Luo A, et al. Protein kinase C and Ca2+ -calmodulin-dependent protein kinase II mediate the enlarged reverse INCX induced by ouabain-increased late sodium current in rabbit ventricular myocytes. Exp Physiol. 2015;100:399–409.
Lipicky RJ, Donovan TJ, Traycoff RB. Ouabain conversion of aconitine-induced atrial tachycardias. Life Sci I. 1971;10:1021–7.
Chen X, Guo H, Li Q, Zhang Y, Liu H, Zhang X, et al. Protective effect of berberine on aconite induced myocardial injury and the associated mechanisms. Mol Med Rep. 2018;18:4468–76.
Vornanen M. The temperature dependence of electrical excitability in fish hearts. J Exp Biol. 2016;219:1941–52.
Hyun NG, Hyun KH, Lee K, Kaang BK. Temperature dependence of action potential parameters in Aplysia neurons. Neurosignals. 2012;20:252–64.
Abramochkin DV, Haverinen J, Mitenkov YA, Vornanen M. Temperature and external K+ dependence of electrical excitation in ventricular myocytes of cod-like fishes. J Exp Biol. 2019;222:jeb193607.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC, 81773828, 81770407, 81903730), the Natural Science Foundation of Hebei Province (C2011206145, H2018206297, C2018206277, H2019423130), and the Research Foundation of Education Bureau of Hebei Province (BJ2019037).
Author information
Authors and Affiliations
Contributions
SWS, CW, and HMW designed the research. XCW, QZJ, YLY, HDW, CTL, XDM, XYC, QFM, and BCG performed the experiments. XCW, YLY, QZJ, HCG, and SWS analyzed the data. XCW, SWS, HMW, and XDM wrote the paper and prepared all of the figures. All authors reviewed and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Xc., Jia, Qz., Yu, Yl. et al. Inhibition of the INa/K and the activation of peak INa contribute to the arrhythmogenic effects of aconitine and mesaconitine in guinea pigs. Acta Pharmacol Sin 42, 218–229 (2021). https://doi.org/10.1038/s41401-020-0467-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0467-6
Keywords
This article is cited by
-
Lysine 2-hydroxyisobutyrylation of HXK1 alters energy metabolism and KATP channel function in the atrium from patients with atrial fibrillation
Cell Communication and Signaling (2025)
-
The pharmacology, toxicology, and detoxification of Aconitum kusnezoffii Reichb., traditional and modern views
Applied Biological Chemistry (2025)