Abstract
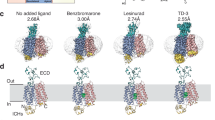
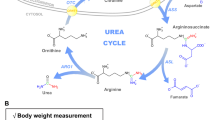
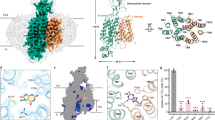
Urate transporter 1 (URAT1) and glucose transporter 9 (GLUT9) are important targets for the development of uric acid-lowering drugs. We previously showed that the flexible linkers of URAT1 inhibitors could enhance their potency. In this study we designed and synthesized CDER167, a novel RDEA3710 analogue, by introducing a linker (methylene) between the naphthalene and pyridine rings to increase flexibility, and characterized its pharmacological and pharmacokinetics properties in vitro and in vivo. We showed that CDER167 exerted dual-target inhibitory effects on both URAT1 and GLUT9: CDER167 concentration-dependently inhibited the uptake of [14C]-uric acid in URAT1-expressing HEK293 cells with an IC50 value of 2.08 ± 0.31 μM, which was similar to that of RDEA3170 (its IC50 value was 1.47 ± 0.23 μM). Using site-directed mutagenesis, we demonstrated that CDER167 might interact with URAT1 at S35 and F365. In GLUT9-expressing HEK293T cells, CDER167 concentration-dependently inhibited GLUT9 with an IC50 value of 91.55 ± 15.28 μM, whereas RDEA3170 at 100 μM had no effect on GLUT9. In potassium oxonate-induced hyperuricemic mice, oral administration of CDER167 (10 mg·kg−1 · d−1) for 7 days was more effective in lowering uric acid in blood and significantly promoted uric acid excretion in urine as compared with RDEA3170 (20 mg·kg−1 · d−1) administered. The animal experiment proved the safety of CDER167. In addition, CDER167 displayed better bioavailability than RDEA3170, better metabolic stability and no hERG toxicity at 100 μM. These results suggest that CDER167 deserves further investigation as a candidate antihyperuricemic drug targeting URAT1 and GLUT9.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
27 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41401-022-00872-z
References
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039–52.
Dalbeth N, Phipps-Green A, House ME, Gamble GD, Horne A, Stamp LK, et al. Body mass index modulates the relationship of sugar-sweetened beverage intake with serum urate concentrations and gout. Arthritis Res Ther. 2015;17:263.
Wu J, Zhang YP, Qu Y, Jie LG, Deng JX, Yu QH. Efficacy of uric acid-lowering therapy on hypercholesterolemia and hypertriglyceridemia in gouty patients. Int J Rheum Dis. 2019;22:1445–51.
Hou YL, Yang XL, Wang CX, Zhi LX, Yang MJ, You CG. Hypertriglyceridemia and hyperuricemia: a retrospective study of urban residents. Lipids Health Dis. 2019;18:81.
Wang Y, Chi J, Che K, Chen Y, Sun X, Wang Y, et al. Fasting plasma glucose and serum uric acid levels in a general Chinese population with normal glucose tolerance: a U-shaped curve. PLoS ONE. 2017;12:e180111.
Huffman JE, Albrecht E, Teumer A, Mangino M, Kapur K, Johnson T, et al. Modulation of genetic associations with serum urate levels by body-mass-index in humans. PLoS ONE. 2015;10:e119752.
Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. 2016;29:3–8.
So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120:1791–9.
Strilchuk L, Fogacci F, Cicero AF. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin Drug Saf. 2019;18:261–71.
Miner JN, Tan PK, Hyndman D, Liu S, Iverson C, Nanavati P, et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther. 2016;18:214.
Hautekeete ML, Henrion J, Naegels S, DeNeve A, Adler M, Deprez C, et al. Severe hepatotoxicity related to benzarone: a report of three cases with two fatalities. Liver. 1995;15:25–9.
Gutman J, Kachur SP, Slutsker L, Nzila A, Mutabingwa T. Combination of probenecid-sulphadoxine-pyrimethamine for intermittent preventive treatment in pregnancy. Malar J. 2012;11:39.
Robinson PC, Dalbeth N. Lesinurad for the treatment of hyperuricaemia in people with gout. Expert Opin Pharmacother. 2017;18:1875–81.
Shen Z, Gillen M, Miner JN, Bucci G, Wilson DM, Hall JW. Pharmacokinetics, pharmacodynamics, and tolerability of verinurad, a selective uric acid reabsorption inhibitor, in healthy adult male subjects. Drug Des Devel Ther. 2017;11:2077–86.
Wu T, Chen J, Dong S, Li H, Cao Y, Tian Y, et al. Identification and characterization of a potent and selective inhibitor of human urate transporter 1. Pharmacol Rep. 2017;69:1103–12.
Bibert S, Hess SK, Firsov D, Thorens B, Geering K, Horisberger JD, et al. Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Ren Physiol. 2009;297:F612–9.
Tian H, Liu W, Zhou Z, Shang Q, Liu Y, Xie Y, et al. Discovery of a flexible triazolylbutanoic acid as a highly potent uric acid transporter 1 (URAT1) Inhibitor. Molecules. 2016;21:1543.
Zhao T, Zhao Z, Lu F, Chang S, Zhang J, Pang J, et al. Two- and three-dimensional QSAR studies on hURAT1 inhibitors with flexible linkers: topomer CoMFA and HQSAR. Mol Divers. 2020;24:141–54.
Chen Y, Zhao Z, Li Y, Yang Y, Li L, Jiang Y, et al. Baicalein alleviates hyperuricemia by promoting uric acid excretion and inhibiting xanthine oxidase. Phytomedicine. 2020;80:153374.
Chen Y, Zhao Z, Li Y, Li L, Jiang Y, Cao Y, et al. Characterizations of the urate transporter, GLUT9, and its potent inhibitors by patch-clamp technique. SLAS. Discovery. 2021;26:450–9.
Nakamura M, Fujita K, Toyoda Y, Nakamura M, Fujita K, Toyoda Y, et al. Investigation of the transport of xanthine dehydrogenase inhibitors by the urate transporter ABCG2. Drug Metab Pharmacokinet. 2018;33:77–81.
Wu XH, Wang CZ, Wang SQ, Chao M, He Y, Zhang J, et al. Anti-hyperuricemia effects of allopurinol are improved by Smilax riparia, a traditional Chinese herbal medicine. J Ethnopharmacol. 2015;162:362–8.
Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307.
Helliwell RM. Recording hERG potassium currents and assessing the effects of compounds using the whole-cell patch-clamp technique. Methods Mol Biol. 2008;491:279–95.
Kamiya K, Niwa R, Morishima M, Honjo H, Sanguinetti MC. Molecular determinants of hERG channel block by terfenadine and cisapride. J Pharm Sci. 2008;108:301–7.
Tan PK, Liu S, Gunic E, Miner JN. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci Rep. 2017;7:665.
Gliozzi M, Malara N, Muscoli S, Mollace V. The treatment of hyperuricemia. Int J Cardiol. 2016;213:23–7.
Taniguchi T, Ashizawa N, Matsumoto K, Saito R, Motoki K, Sakai M, et al. Pharmacological evaluation of dotinurad, a selective urate reabsorption inhibitor. J Pharmacol Exp Ther. 2019;371:162–70.
Tan PK, Ostertag TM, Miner JN. Mechanism of high affinity inhibition of the human urate transporter URAT1. Sci Rep. 2016;6:34995.
Woodward OM. ABCG2: the molecular mechanisms of urate secretion and gout. Am J Physiol Ren Physiol. 2015;309:F485–8.
Miyata H, Takada T, Toyoda Y, Matsuo H, Ichida K, Suzuki H. Identification of febuxostat as a new strong ABCG2 inhibitor: potential applications and risks in clinical situations. Front Pharmacol. 2016;7:518.
Qin Z, Wang S, Lin Y, Zhao Y, Yang S, Song J, et al. Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharm Sin B. 2018;8:306–15.
Chen M, Ye C, Zhu J, Zhang P, Jiang Y, Lu X, et al. Bergenin as a novel urate-lowering therapeutic strategy for hyperuricemia. Front Cell Dev Biol. 2020;8:703.
Torralba KD, De Jesus E, Rachabattula S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: current and future directions. Int J Rheum Dis. 2012;15:499–506.
Kedar E, Simkin PA. A perspective on diet and gout. Adv Chronic Kidney Dis. 2012;19:392–7.
Mehmood A, Zhao L, Wang C, Nadeem M, Raza A, Ali N, et al. Management of hyperuricemia through dietary polyphenols as a natural medicament: a comprehensive review. Crit Rev Food Sci Nutr. 2019;59:1433–55.
Major TJ, Dalbeth N, Stahl EA, Merriman TR. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2018;14:341–53.
Auberson M, Stadelmann S, Stoudmann C, Seuwen K, Koesters R, Thorens B, et al. SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney. Pflug Arch. 2018;470:1739–51.
Ruiz A, Gautschi I, Schild L, Bonny O. Human mutations in SLC2A9 (Glut9) affect transport capacity for urate. Front Physiol. 2018;9:476.
Kaufmann P, Torok M, Hanni A, Roberts P, Gasser R, Krahenbuhl S, et al. Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology. 2005;41:925–35.
Reinders MK, van Roon EN, Jansen TL, Delsing J, Griep EN, Hoekstra M, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis. 2009;68:51–6.
Fleischmann R, Kerr B, Yeh LT, Suster M, Shen Z, Polvent E, et al. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatol (Oxf). 2014;53:2167–74.
Hall J, Gillen M, Yang X, Shen Z. Pharmacokinetics, pharmacodynamics, and tolerability of concomitant administration of verinurad and febuxostat in healthy male volunteers. Clin Pharmacol Drug Dev. 2019;8:179–87.
Fleischmann R, Winkle P, Hall J, Valdez S, Liu S, Yan X, et al. Pharmacodynamic and pharmacokinetic effects and safety of verinurad in combination with febuxostat in adults with gout: a phase IIa, open-label study. RMD Open. 2018;4:e647.
Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharmacol Res. 2007;24:450–70.
Yan N. Structural biology of the major facilitator superfamily transporters. Annu Rev Biophys. 2015;44:257–83.
Li DC, Nichols CG, Sala-Rabanal M. Role of a hydrophobic pocket in polyamine interactions with the polyspecific organic cation transporter OCT3. J Biol Chem. 2015;290:27633–43.
Lu J, Dalbeth N, Yin H, Li C, Merriman TR, Wei WH, et al. Mouse models for human hyperuricaemia: a critical review. Nat Rev Rheumatol. 2019;15:413–26.
Alghamdi YS, Soliman MM, Nassan MA. Impact of Lesinurad and allopurinol on experimental hyperuricemia in mice: biochemical, molecular and immunohistochemical study. BMC Pharmacol Toxicol. 2020;21:10.
Tan Y, Wang L, Gao J, Ma J, Yu H, Zhang Y, et al. Multiomics integrative analysis for discovering the potential mechanism of dioscin against hyperuricemia mice. J Proteome Res. 2020;20:645–60.
Chen M, Lu X, Lu C, Shen N, Jiang Y, Chen M, et al. Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway. Arthritis Res Ther. 2018;20:20.
Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin H, et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem. 2004;279:45942–50.
Srivastava S, Nakagawa K, He X, Kimura T, Fukutomi T, Miyauchi S, et al. Identification of the multivalent PDZ protein PDZK1 as a binding partner of sodium-coupled monocarboxylate transporter SMCT1 (SLC5A8) and SMCT2 (SLC5A12). J Physiol Sci. 2019;69:399–408.
Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–45.
Yano H, Tamura Y, Kobayashi K, Tanemoto M, Uchida S. Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease. Clin Exp Nephrol. 2014;18:50–5.
Sekine T, Endou H. The mechanisms of urate transport in the kidney and the intestine. Nihon Rinsho. 1996;54:3237–42.
Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Asp Med. 2013;34:413–35.
Xu X, Li C, Zhou P, Jiang T. Uric acid transporters hiding in the intestine. Pharm Biol. 2016;54:3151–5.
Hoque KM, Dixon EE, Lewis RM, Allan J, Gamble GD, Phipps-Green AJ, et al. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat Commun. 2020;11:2767.
DeBosch BJ, Kluth O, Fujiwara H, Schurmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun. 2014;5:4642.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81773794 and 81974507) and the Natural Science Foundation of Guangdong Province (2018A0303130088).
Author information
Authors and Affiliations
Contributions
JXP, YXT and JYG conceived and designed the research. ZAZ, YJ, YYC and QSL conducted the experiments. LL, YML YC and CTL analyzed the results. TW, YY and PZZ wrote the paper. All authors read and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, Za., Jiang, Y., Chen, Yy. et al. CDER167, a dual inhibitor of URAT1 and GLUT9, is a novel and potent uricosuric candidate for the treatment of hyperuricemia. Acta Pharmacol Sin 43, 121–132 (2022). https://doi.org/10.1038/s41401-021-00640-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00640-5
Keywords
This article is cited by
-
Serum urate, cardiovascular mediators, and atrial fibrillation: genetic evidence for URAT1-targeted therapy
Clinical Rheumatology (2026)
-
URAT1 and GLUT9 as drug targets in gout: progress in transporter-directed therapies and delivery technologies
Inflammopharmacology (2025)
-
Anti-hyperuricemia effect of Clerodendranthus spicatus: a molecular biology study combined with metabolomics
Scientific Reports (2024)
-
Emerging Urate-Lowering Drugs and Pharmacologic Treatment Strategies for Gout: A Narrative Review
Drugs (2023)