Abstract
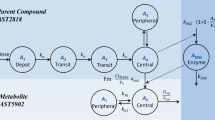
Furmonertinib was designed for the treatment of non-small cell lung cancer (NSCLC) patients with EGFR T790M mutation. In this study, we investigated the metabolic disposition and mass balance in humans and tissue distribution in rats. After a single oral administration of 97.9 μCi/81.5 mg [14C]-furmonertinib mesylate to six healthy male volunteers, the absorption process of furmonertinib was fast with a tmax of total plasma radioactivity at 0.75 h. Afterward, furmonertinib was extensively metabolized, with the parent drug and active metabolite AST5902 accounting for 1.68% and 0.97% of total radioactivity in plasma. The terminal t1/2 of total radioactivity in plasma was as long as 333 h, suggesting that the covalent binding of drug-related substances to plasma proteins was irreversible to a great extent. The most abundant metabolites identified in feces were desmethyl metabolite (AST5902), cysteine conjugate (M19), and parent drug (M0), which accounted for 6.28%, 5.52%, and 1.38% of the dose, respectively. After intragastric administration of 124 μCi/9.93 mg/kg [14C]-furmonertinib to rats, drug-related substances were widely and rapidly distributed in tissues within 4 h. The concentration of total radioactivity in the lung was 100-fold higher than that in rat plasma, which could be beneficial to the treatment of lung cancer. Mass balance in humans was achieved with 77.8% of the administered dose recovered in excretions within 35 days after administration, including 6.63% and 71.2% in urine and feces, respectively. In conclusion, [14C]-furmonertinib is completely absorbed and rapidly distributed into lung tissue, extensively metabolized in humans, presented mostly as covalent conjugates in plasma, and slowly eliminated mostly via fecal route.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34.
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–7.
Han W, Du Y. Recent development of the second and third generation irreversible epidermal growth factor receptor inhibitors. Chem Biodivers. 2017; 14. https://doi.org/10.1002/cbdv.201600372.
Murtuza A, Bulbul A, Shen JP, Keshavarzian P, Woodward BD, Lopez-Diaz FJ, et al. Novel third-generation EGFR tyrosine kinase inhibitors and strategies to overcome therapeutic resistance in lung cancer. Cancer Res. 2019;79:689–98.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
Kim ES. Olmutinib: first global approval. Drugs. 2016;76:1153–7.
Shi Y, Zhang S, Hu X, Feng J, Ma Z, Zhou J, et al. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC with EGFR T790M mutation. J Thorac Oncol. 2020;15:1015–26.
Liu X, Li W, Zhang Y, Jiang Y, Zhao Q, Zhong D. Simultaneous determination of alflutinib and its active metabolite in human plasma using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2019;176:112735.
Graham RA, Lum BL, Morrison G, Chang I, Jorga K, Dean B, et al. A single dose mass balance study of the Hedgehog pathway inhibitor vismodegib (GDC-0449) in humans using accelerator mass spectrometry. Drug Metab Dispos. 2011;39:1460–7.
Liu X, Feng D, Zheng M, Zhong D. Characterization of covalent binding to human plasma proteins by selected covalent tyrisine kinase inhibitors. Drug Metab Pharmacokinet. 2020;35:456–65.
Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Chem Biol. 2011;10:307–17.
Meng J, Liu XY, Ma S, Zhang H, Yu SD, Zhang YF, et al. Metabolism and disposition of pyrotinib in healthy male volunteers: covalent binding with human plasma protein. Acta Pharmacol Sin. 2019;40:980–8.
Wang J, Li-Chan XX, Atherton J, Deng L, Espina R, Yu L, et al. Characterization of HKI-272 covalent binding to human serum albumin. Drug Metab Dispos. 2010;38:1083–93.
Dickinson PA, Cantarini MV, Collier J, Frewer P, Martin S, Pickup K, et al. Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab Dispos. 2016;44:1201–12.
Scheers E, Leclercq L, de Jong J, Bode N, Bockx M, Laenen A, et al. Absorption, metabolism and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug Metab Dispos. 2014;43:289–97.
Liu X, Guo Z, Chen Z, Zhang Y, Zhou J, Jiang Y, et al. Alflutinib (AST2818), primarily metabolized by CYP3A4, is a potent CYP3A4 inducer. Acta Pharmacol Sin. 2020;41:1366–76.
Acknowledgements
This article was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA12050306) and the National Natural Science Foundation of China (No. 81521005).
Author information
Authors and Affiliations
Contributions
JM, ZDC, and DFZ contributed to the writing of this paper. JM, XYL, and DFZ participated in the drug metabolism study. HZ and LYM participated in clinical trials. JJB and YJ conducted pharmacological experiments. ZDC and YFZ performed pharmaceutical analysis and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Meng, J., Zhang, H., Bao, Jj. et al. Metabolic disposition of the EGFR covalent inhibitor furmonertinib in humans. Acta Pharmacol Sin 43, 494–503 (2022). https://doi.org/10.1038/s41401-021-00667-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00667-8
Keywords
This article is cited by
-
Pharmacokinetics, mass balance, and metabolism of [14C]PLB1004, a selective and irreversible EGFR-TKI in humans
Cancer Chemotherapy and Pharmacology (2025)
-
Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials
Signal Transduction and Targeted Therapy (2023)
-
Effect of autoinduction and food on the pharmacokinetics of furmonertinib and its active metabolite characterized by a population pharmacokinetic model
Acta Pharmacologica Sinica (2022)
-
Furmonertinib: First Approval
Drugs (2021)