Abstract
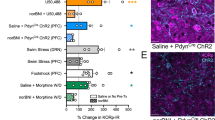
Major depression disorder is a severe and recurrent neuropsychological disorder characterized by lowered mood and social activity and cognitive impairment. Owing to unclear molecular mechanisms of depression, limited interventions are available in clinic. In this study we investigated the role of dynorphin/κ opioid receptor system in the development of depression. Mice were subjected to chronic social defeat stress for 14 days. Chronic social defeat stress induced significant social avoidance in mice characterized by decreased time duration in the interaction zone and increased time duration in the corner zone. Pre-administration of a κ opioid receptor antagonist norBNI (10 mg/kg, i.p.) could prevent the development of social avoidance induced by chronic social defeat stress. Social avoidance was not observed in κ opioid receptor knockout mice subjected to chronic social defeat stress. We further revealed that social defeat stress activated c-fos and ERK signaling in the amygdala without affecting the NAc, hippocampus and hypothalamus, and ERK activation was blocked by systemic injection of norBNI. Finally, the expression of dynorphin A, the endogenous ligand of κ opioid receptor, was significantly increased in the amygdala following social defeat stress; microinjection of norBNI into the amygdala prevented the development of depressive-like behaviors caused by social defeat stress. The present study demonstrates that upregulated dynorphin/κ opioid receptor system in the amygdala leads to the emergence of depression following chronic social defeat stress, and sheds light on κ opioid receptor antagonists as potential therapeutic agents for the prevention and treatment of depression following chronic stress.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
18 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41401-022-01016-z
References
Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neurosci Biobehav Rev. 2016;69:313–32.
Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319.
Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
Lima-Ojeda JM, Rupprecht R, Baghai TC. Neurobiology of depression: a neurodevelopmental approach. World J Biol Psychiatry. 2018;19:349–59.
Czeh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Hollis F, Kabbaj M. Social defeat as an animal model for depression. ILAR J. 2014;55:221–32.
Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull. 2010;26:327–37.
Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–9.
Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY. Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther. 2011;339:438–50.
Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–5.
Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–23.
Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35:12917–31.
Carlezon WA Jr., Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009;123:334–43.
Knoll AT, Carlezon WA Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73.
Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206.
Hang A, Wang YJ, He L, Liu JG. The role of the dynorphin/kappa opioid receptor system in anxiety. Acta Pharmacol Sin. 2015;36:783–90.
Wang YH, Sun JF, Tao YM, Chi ZQ, Liu JG. The role of kappa-opioid receptor activation in mediating antinociception and addiction. Acta Pharmacol Sin. 2010;31:1065–70.
Belmaker RH. The future of depression psychopharmacology. CNS Spectr. 2008;13:682–7.
Kumar U, Medel-Matus JS, Redwine HM, Shin D, Hensler JG, Sankar R, et al. Effects of selective serotonin and norepinephrine reuptake inhibitors on depressive- and impulsive-like behaviors and on monoamine transmission in experimental temporal lobe epilepsy. Epilepsia. 2016;57:506–15.
Ball SG, Kuhn A, Wall D, Shekhar A, Goddard AW. Selective serotonin reuptake inhibitor treatment for generalized anxiety disorder: a double-blind, prospective comparison between paroxetine and sertraline. J Clin Psychiatry. 2005;66:94–9.
Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511.
Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, et al. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci. 2012;32:17582–96.
Sundaramurthy S, Annamalai B, Samuvel DJ, Shippenberg TS, Jayanthi LD, Ramamoorthy S. Modulation of serotonin transporter function by kappa-opioid receptor ligands. Neuropharmacology. 2017;113:281–92.
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25.
Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–314.
Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR, et al. An amygdala-hippocampus subnetwork that encodes variation in human mood. Cell. 2018;175:1688–700 e14.
Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–83.
Todorovic C, Sherrin T, Pitts M, Hippel C, Rayner M, Spiess J. Suppression of the MEK/ERK signaling pathway reverses depression-like behaviors of CRF2-deficient mice. Neuropsychopharmacology. 2009;34:1416–26.
Wang JQ, Mao L. The ERK pathway: molecular mechanisms and treatment of depression. Mol Neurobiol. 2019;56:6197–205.
Schneider F, Weiss U, Kessler C, Muller-Gartner HW, Posse S, Salloum JB, et al. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry. 1999;45:863–71.
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8.
Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett. 2002;328:233–6.
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209.
Staugaard SR. Threatening faces and social anxiety: a literature review. Clin Psychol Rev. 2010;30:669–90.
Varlinskaya EI, Johnson JM, Przybysz KR, Deak T, Diaz MR. Adolescent forced swim stress increases social anxiety-like behaviors and alters kappa opioid receptor function in the basolateral amygdala of male rats. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109812.
Schwarzer C. 30 years of dynorphins–new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123:353–70.
Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, et al. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J Pharmacol Exp Ther. 2013;346:545–54.
Wang YJ, Hang A, Lu YC, Long Y, Zan GY, Li XP, et al. Kappa opioid receptor activation in different brain regions differentially modulates anxiety-related behaviors in mice. Neuropharmacology. 2016;110:92–101.
Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–76.
Hing B, Braun P, Cordner ZA, Ewald ER, Moody L, McKane M, et al. Chronic social stress induces DNA methylation changes at an evolutionary conserved intergenic region in chromosome X. Epigenetics. 2018;13:627–41.
Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224.
Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter 8:Unit 8.10A. https://doi.org/10.1002/0471142301.ns0810as14.
Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, et al. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–9.
Potter DN, Damez-Werno D, Carlezon WA Jr., Cohen BM, Chartoff EH. Repeated exposure to the kappa-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry. 2011;70:744–53.
Iniguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–55.
Mezuk B, Golden SH, Eaton WW, Lee HB. Depression and body composition among older adults. Aging Ment Health. 2012;16:167–72.
Barlow J. Antenatal anxiety, parenting and behavioural/emotional problems in children. Br J Psychiatry. 2002;181:440–1.
Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. Am Psychol. 2006;61:10–26.
Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, Trainor BC. Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:166–74.
Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA Jr. Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol. 2015;26:654–63.
Hu E, Mueller E, Oliviero S, Papaioannou VE, Johnson R, Spiegelman BM. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–103.
Numa C, Nagai H, Taniguchi M, Nagai M, Shinohara R, Furuyashiki T. Social defeat stress-specific increase in c-Fos expression in the extended amygdala in mice: Involvement of dopamine D1 receptor in the medial prefrontal cortex. Sci Rep. 2019;9:16670.
Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13.
Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528.
Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–33.
Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, et al. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–83.
Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berlin). 2010;210:137–47.
Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–22.
Galeotti N, Ghelardini C. Regionally selective activation and differential regulation of ERK, JNK and p38 MAP kinase signalling pathway by protein kinase C in mood modulation. Int J Neuropsychopharmacol. 2012;15:781–93.
Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–32.
Gilpin NW, Roberto M, Koob GF, Schweitzer P. Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology. 2014;77:294–302.
Simmons SC, Shepard RD, Gouty S, Langlois LD, Flerlage WJ, Cox BM, et al. Early life stress dysregulates kappa opioid receptor signaling within the lateral habenula. Neurobiol Stress. 2020;13:100267.
Tejeda HA, Wu J, Kornspun AR, Pignatelli M, Kashtelyan V, Krashes MJ, et al. Pathway- and cell-specific kappa-Opioid receptor modulation of excitation-inhibition balance differentially gates D1 and D2 accumbens neuron activity. Neuron. 2017;93:147–63.
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90.
Fakhoury M. Revisiting the Serotonin Hypothesis: Implications for major depressive disorders. Mol Neurobiol. 2016;53:2778–86.
Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–64.
Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517.
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81:886–97.
Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16:472–86.
Kozinn J, Mao L, Arora A, Yang L, Fibuch EE, Wang JQ. Inhibition of glutamatergic activation of extracellular signal-regulated protein kinases in hippocampal neurons by the intravenous anesthetic propofol. Anesthesiology. 2006;105:1182–91.
Acknowledgements
This research was supported by the National Natural Science Foundation of China 81130087, 81671322, 82030112 (to JGL), 81771188 (to ZQL), 81773710 (to YJW), 81801321 (to GYZ), by the Science and Technology Commission of Shanghai Municipality 19401930500 (to ZQL), by the Youth Innovation Promotion Association of the Chinese Academy of Sciences 2017334 (to YJW), and by the China Postdoctoral Science Foundation 2018M640423 (to GYZ). The authors would like to thank Dr. Xin Xie, Shanghai Institute of Materia Medica, for providing KOR knockout mice with detailed gene phenotype identification protocol.
Author information
Authors and Affiliations
Contributions
ZQL, QLL, and GYZ designed the experiments. GYZ and XS performed the experiments with the assistance of YJW, RL, CYW, JRC, and LBG. GYZ and XS performed data statistical analysis. GYZ wrote the manuscript and JGL, ZQL, QLL, and WJD revised it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zan, Gy., Sun, X., Wang, Yj. et al. Amygdala dynorphin/κ opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress. Acta Pharmacol Sin 43, 577–587 (2022). https://doi.org/10.1038/s41401-021-00677-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00677-6
Keywords
This article is cited by
-
Astroglial kappa opioid receptor-mediated reduction of glutamate exchanger xCT in the prelimbic cortex underlies chronic stress-induced depressive-like behaviors
Molecular Psychiatry (2025)
-
The Dynorphin/-Opioid Receptor System at the Interface of Hyperalgesia/Hyperkatifeia and Addiction
Current Addiction Reports (2025)
-
The Antidepressant Effect of Magnolol on Depression-Like Behavior of CORT-Treated Mice
Journal of Molecular Neuroscience (2024)
-
Dynorphin participates in interaction between depression and non-erosive reflux disease
Esophagus (2023)