Abstract
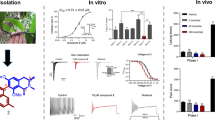
Voltage-gated sodium channel Nav1.7 robustly expressed in peripheral nociceptive neurons has been considered as a therapeutic target for chronic pain, but there is no selective Nav1.7 inhibitor available for therapy of chronic pain. Ralfinamide has shown anti-nociceptive activity in animal models of inflammatory and neuropathic pain and is currently under phase III clinical trial for neuropathic pain. Based on ralfinamide, a novel small molecule (S)-2-((3-(4-((2-fluorobenzyl) oxy) phenyl) propyl) amino) propanamide (QLS-81) was synthesized. Here, we report the electrophysiological and pharmacodynamic characterization of QLS-81 as a Nav1.7 channel inhibitor with promising anti-nociceptive activity. In whole-cell recordings of HEK293 cells stably expressing Nav1.7, QLS-81 (IC50 at 3.5 ± 1.5 μM) was ten-fold more potent than its parent compound ralfinamide (37.1 ± 2.9 μM) in inhibiting Nav1.7 current. QLS-81 inhibition on Nav1.7 current was use-dependent. Application of QLS-81 (10 μM) caused a hyperpolarizing shift of the fast and slow inactivation of Nav1.7 channel about 7.9 mV and 26.6 mV, respectively, and also slowed down the channel fast and slow inactivation recovery. In dissociated mouse DRG neurons, QLS-81 (10 μM) inhibited native Nav current and suppressed depolarizing current pulse-elicited neuronal firing. Administration of QLS-81 (2, 5, 10 mg· kg−1· d−1, i.p.) in mice for 10 days dose-dependently alleviated spinal nerve injury-induced neuropathic pain and formalin-induced inflammatory pain. In addition, QLS-81 (10 μM) did not significantly affect ECG in guinea pig heart ex vivo; and administration of QLS-81 (10, 20 mg/kg, i.p.) in mice had no significant effect on spontaneous locomotor activity. Taken together, our results demonstrate that QLS-81, as a novel Nav1.7 inhibitor, is efficacious on chronic pain in mice, and it may hold developmental potential for pain therapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Xu L, Ding X, Wang T, Mou S, Sun H, Hou T. Voltage-gated sodium channels: structures, functions, and molecular modeling. Drug Discov Today. 2019;24:1389–97.
Minett MS, Nassar MA, Clark AK, Passmore G, Dickenson AH, Wang F, et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun. 2012;3:791.
Choi JS, Boralevi F, Brissaud O, Sanchez-Martin J, Te Morsche RH, Dib-Hajj SD, et al. Paroxysmal extreme pain disorder: a molecular lesion of peripheral neurons. Nat Rev Neurol. 2011;7:51–5.
Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32.
Gardella E, Becker F, Moller RS, Schubert J, Lemke JR, Larsen LH, et al. Benign infantile seizures and paroxysmal dyskinesia caused by an SCN8A mutation. Ann Neurol. 2016;79:428–36.
Liu Y, Wang K. Exploiting the diversity of ion channels: modulation of ion channels for therapeutic indications. Handb Exp Pharmacol. 2019;260:187–205.
Alexandrou AJ, Brown AR, Chapman ML, Estacion M, Turner J, Mis MA, et al. Subtype-selective small molecule inhibitors reveal a fundamental role for Nav1.7 in nociceptor electrogenesis, axonal conduction and presynaptic release. PLoS One 2016;11:e0152405.
Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2013;14:49–62.
Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–74.
Waxman SG, Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005;11:555–62.
Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171–4.
Baker MD, Nassar MA. Painful and painless mutations of SCN9A and SCN11A voltage-gated sodium channels. Pflug Arch. 2020;472:865–80.
Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–8.
Theile JW, Jarecki BW, Piekarz AD, Cummins TR. Nav1.7 mutations associated with paroxysmal extreme pain disorder, but not erythromelalgia, enhance Navbeta4 peptide-mediated resurgent sodium currents. J Physiol. 2011;589:597–608.
McDermott LA, Weir GA, Themistocleous AC, Segerdahl AR, Blesneac I, Baskozos G, et al. Defining the functional role of NaV1.7 in human nociception. Neuron. 2019;101:905–19.e8.
Alrashood ST. Carbamazepine. Profiles Drug Subst Excip Relat Methodol. 2016;41:133–321.
Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123:335–49.
Singh S, Kerndt C, Zeltser R. Mexiletine. In: StatPearls. FL: Treasure Island; 2020.
Mulroy MF. Systemic toxicity and cardiotoxicity from local anesthetics: incidence and preventive measures. Reg Anesth Pain Med. 2002;27:556–61.
Nguyen PT, DeMarco KR, Vorobyov I, Clancy CE, Yarov-Yarovoy V. Structural basis for antiarrhythmic drug interactions with the human cardiac sodium channel. Proc Natl Acad Sci USA. 2019;116:2945–54.
Sun S, Jia Q, Zenova AY, Wilson MS, Chowdhury S, Focken T, et al. Identification of selective acyl sulfonamide-cycloalkylether inhibitors of the voltage-gated sodium channel (Nav) 1.7 with potent analgesic activity. J Med Chem. 2019;62:908–27.
Liang X, Yu G, Su R. Effects of ralfinamide in models of nerve injury and chemotherapy-induced neuropathic pain. Eur J Pharmacol. 2018;823:27–34.
Stummann TC, Salvati P, Fariello RG, Faravelli L. The anti-nociceptive agent ralfinamide inhibits tetrodotoxin-resistant and tetrodotoxin-sensitive Na+ currents in dorsal root ganglion neurons. Eur J Pharmacol. 2005;510:197–208.
Veneroni O, Maj R, Calabresi M, Faravelli L, Fariello RG, Salvati P. Anti-allodynic effect of NW-1029, a novel Na+ channel blocker, in experimental animal models of inflammatory and neuropathic pain. Pain. 2003;102:17–25.
Zhang SH, Blech-Hermoni Y, Faravelli L, Seltzer Z. Ralfinamide administered orally before hindpaw neurectomy or postoperatively provided long-lasting suppression of spontaneous neuropathic pain-related behavior in the rat. Pain. 2008;139:293–305.
Colombo E, Curatolo L, Caccia C, Salvati P, Faravelli L. 344 ralfinamide acts through nmda receptor complex: a central role for chronic pain treatment. Eur J Pain. 2007;11:S152–S3.
Du X, Hao H, Gigout S, Huang D, Yang Y, Li L, et al. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain. 2014;155:2306–22.
Andersen PL, Doucette JR, Nazarali AJ. A novel method of eliminating non-neuronal proliferating cells from cultures of mouse dorsal root ganglia. Cell Mol Neurobiol. 2003;23:205–10.
Bourquin AF, Suveges M, Pertin M, Gilliard N, Sardy S, Davison AC, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14.
Wang X, Zhang G, Qiao Y, Feng C, Zhao X. Crocetin attenuates spared nerve injury-induced neuropathic pain in mice. J Pharmacol Sci. 2017;135:141–7.
Bonin RP, Bories C, De, Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26.
Boccella S, Guida F, Palazzo E, Marabese I, de Novellis V, Maione S, et al. Spared nerve injury as a long-lasting model of neuropathic pain. Methods Mol Biol. 2018;1727:373–8.
Harton LR, Richardson JR, Armendariz A, Nazarian A. Dissociation of morphine analgesic effects in the sensory and affective components of formalin-induced spontaneous pain in male and female rats. Brain Res. 2017;1658:36–41.
Zhang Y, Peng D, Huang B, Yang Q, Zhang Q, Chen M, et al. Discovery of a novel Nav1.7 inhibitor from cyriopagopus albostriatus venom with potent analgesic efficacy. Front Pharmacol. 2018;9:1158.
Qiu B, Wang Y, Li C, Guo H, Xu Y. Utility of the JT peak interval and the JT area in determining the proarrhythmic potential of QT-shortening agents. J Cardiovasc Pharmacol Ther. 2019;24:160–71.
Wang Y, Mi J, Lu K, Lu Y, Wang K. Comparison of gating properties and use-dependent block of Nav1.5 and Nav1.7 channels by anti-arrhythmics mexiletine and lidocaine. PLoS One. 2015;10:e0128653.
Ghovanloo MR, Aimar K, Ghadiry-Tavi R, Yu A, Ruben PC. Physiology and pathophysiology of sodium channel inactivation. Curr Top Membr. 2016;78:479–509.
Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–9.
Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F. Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflug Arch. 2010;460:239–48.
Huang S, Zhang W, Chang X, Guo J. Overlap of periodic paralysis and paramyotonia congenita caused by SCN4A gene mutations two family reports and literature review. Channels. 2019;13:110–9.
Song W, Shou W. Cardiac sodium channel Nav1.5 mutations and cardiac arrhythmia. Pediatr Cardiol. 2012;33:943–9.
Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science. 2015;350:aac5464.
Bosmans F, Swartz KJ. Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol Sci. 2010;31:175–82.
de Lera Ruiz M, Kraus RL. Voltage-gated sodium channels: structure, function, pharmacology, and clinical indications. J Med Chem. 2015;58:7093–118.
Murray JK, Ligutti J, Liu D, Zou A, Poppe L, Li H, et al. Engineering potent and selective analogues of GpTx-1, a tarantula venom peptide antagonist of the Nav1.7 sodium channel. J Med Chem. 2015;58:2299–314.
Acknowledgements
This work is supported by research grants from the Science and Technology Program of Guangdong (2018B030334001), the Ministry of Science and Technology of China (2018ZX09711001-004-006) awarded to KWW, and the National Natural Science Foundation of Shandong Province (ZR2020QH100).
Author information
Authors and Affiliations
Contributions
YNL, ARZ, and KWW conceived and designed the research; HLN, YNL, LYD, JW, and HJL performed experiments; DQX and YLZ designed and synthesized compound QLS-81; HLN and YNL analyzed data, prepared figures, and drafted manuscript; YNL, LMS, and KWW edited and revised manuscript; YNL, LMS, and KWW approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Niu, Hl., Liu, Yn., Xue, Dq. et al. Inhibition of Nav1.7 channel by a novel blocker QLS-81 for alleviation of neuropathic pain. Acta Pharmacol Sin 42, 1235–1247 (2021). https://doi.org/10.1038/s41401-021-00682-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00682-9
Keywords
This article is cited by
-
Long noncoding RNA small nucleolar RNA host gene 5 facilitates neuropathic pain in spinal nerve injury by promoting SCN9A expression via CDK9
Human Cell (2024)
-
Naphthylisoquinoline alkaloids, a new structural template inhibitor of Nav1.7 sodium channel
Acta Pharmacologica Sinica (2023)
-
Suberoylanilide Hydroxamic Acid Ameliorates Pain Sensitization in Central Neuropathic Pain After Spinal Cord Injury via the HDAC5/NEDD4/SCN9A Axis
Neurochemical Research (2023)