Abstract
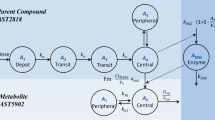
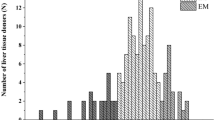
Furmonertinib (Alflutinib, AST2818), as a third-generation epidermal growth factor receptor inhibitor with an advanced efficacy and a relatively wide safety window, has been commercially launched in China recently. However, previous clinical studies demonstrated its time- and dose-dependent clearance in a multiple-dose regimen. In vitro drug metabolism and pharmacokinetic studies have suggested that furmonertinib is mainly metabolized by cytochrome P450 3A4 (CYP3A4) and can induce these enzymes via an increased mRNA expression. This study investigated two important evaluation criteria of CYP3A4 induction by furmonertinib through quantitative proteomics and probe metabolite formation: simultaneous (1) protein expression and (2) enzyme activity with sandwich-cultured primary human hepatocytes in the same well of cell culture plates. Results confirmed that furmonertinib was a potent CYP3A4 inducer comparable with rifampin and could be used as a positive model drug in in vitro studies to evaluate the induction potential of other drug candidates in preclinical studies. In addition, inconsistencies were observed between the protein expression and enzyme activities of CYP3A4 in cells induced by rifampin but not in groups treated with furmonertinib. As such, furmonertinib could be an ideal positive control in the evaluation of CYP3A4 induction. The cells treated with 10 µM rifampin expressed 20.16 ± 5.78 pmol/mg total protein, whereas the cells induced with 0.5 µM furmonertinib expressed 4.8 ± 0.66 pmol/mg protein compared with the vehicle (0.1% dimethyl sulfoxide), which contained 0.65 ± 0.45 pmol/mg protein. The fold change in the CYP3A4 enzyme activity in the cells treated with rifampin was 5.22 ± 1.13, which was similar to that of 0.5 µM furmonertinib (3.79 ± 0.52).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shi Y, Zhang S, Hu X, Feng J, Ma Z, Zhou J, et al. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC with EGFR T790M mutation. J Thorac Oncol. 2020;15:1015–26.
Liu XY, Guo ZT, Chen ZD, Zhang YF, Zhou JL, Jiang Y, et al. Alflutinib (AST2818), primarily metabolized by CYP3A4, is a potent CYP3A4 inducer. Acta Pharmacol Sin. 2020;41:1366–76.
Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmcol. 1999;56:1329–39.
Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62:1238–49.
Williamson BL, Purkayastha S, Hunter CL, Nuwaysir L, Hill J, Easterwood L, et al. Quantitative protein determination for CYP induction via LC-MS/MS. Proteomics. 2011;11:33–41.
Deo AK, Prasad B, Balogh L, Lai Y, Unadkat JD. Interindividual variability in hepatic expression of the multidrug resistance-associated protein 2 (MRP2/ABCC2): quantification by liquid chromatography/tandem mass spectrometry. Drug Metab Dispos. 2012;40:852–5.
Prasad B, Lai Y, Lin Y, Unadkat JD. Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci. 2013;102:787–93.
Gröer C, Busch D, Patrzyk M, Beyer K, Busemann A, Heidecke CD, et al. Absolute protein quantification of clinically relevant cytochrome P450 enzymes and UDP-glucuronosyltransferases by mass spectrometry-based targeted proteomics. J Pharm Biomed Anal. 2014;100:393–401.
Prasad B, Evers R, Gupta A, Hop CE, Salphati L, Shukla S, et al. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42:78–88.
Prasad B, Unadkat JD. Optimized approaches for quantification of drug transporters in tissues and cells by MRM proteomics. AAPS J. 2014;16:634–48.
Savaryn JP, Liu N, Sun J, Ma J, Stresser DM, Jenkins G. Enrichment-free high-throughput liquid chromatography-multiple-reaction monitoring quantification of cytochrome P450 proteins in plated human hepatocytes direct from 96-well plates enables routine protein induction measurements. Drug Metab Dispos. 2020;48:594–602.
Wu SY, Cui SC, Wang L, Zhang YT, Yan XX, Lu HL, et al. 18β-Glycyrrhetinic acid protects against alpha-naphthylisothiocyanate-induced cholestasis through activation of the Sirt1/FXR signaling pathway. Acta Pharmacol Sin. 2018;39:1865–73.
Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KL. Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 1999;289:1592–9.
Xue Y, Deng Q, Zhang Q, Ma Z, Chen B, Yu X, et al. Gigantol ameliorates CCl4-induced liver injury via preventing activation of JNK/cPLA2/12-LOX inflammatory pathway. Sci Rep. 2020;10:22265.
Kawakami H, Ohtsuki S, Kamiie J, Suzuki T, Abe T, Terasaki T. Simultaneous absolute quantification of 11 cytochrome P450 isoforms in human liver microsomes by liquid chromatography tandem mass spectrometry with in silico target peptide selection. J Pharm Sci. 2011;100:341–52.
Kocarek TA, Schuetz EG, Strom SC, Fisher RA, Guzelian PS. Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab Dispos. 1995;23:415–21.
Lu C, Li AP. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague–Dawley rat, minipig, and beagle dog. Chem -Biol Interact. 2001;134:271–81.
Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–94.
Richert L, Tuschl G, Abadie C, Blanchard N, Pekthong D, Mantion G, et al. Use of mRNA expression to detect the induction of drug metabolising enzymes in rat and human hepatocytes. Toxicol Appl Pharmcol. 2009;235:86–96.
Kehinde I, Khan R, Nlooto M, Gordon M. Modulatory influences of antiviral bioactive compounds on cell viability, mRNA and protein expression of cytochrome P450 3A4 and P-glycoprotein in HepG2 and HEK293 cells. Bioorg Chem. 2020;107:104573.
Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–46.
Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207.
Rauh M. LC-MS/MS for protein and peptide quantification in clinical chemistry. J Chromatogr B Anal Technol Biomed Life Sci. 2012;883-884:59–67.
Prasad B, Vrana M, Mehrotra A, Johnson K, Bhatt DK. The promises of quantitative proteomics in precision medicine. J Pharm Sci. 2017;106:738–44.
Vrana M, Whittington D, Nautiyal V, Prasad B. Database of optimized proteomic quantitative methods for human drug disposition-related proteins for applications in physiologically based pharmacokinetic modeling. CPT Pharmacomet Syst Pharmacol. 2017;6:267–76.
Bhatt DK, Prasad B. Critical issues and optimized practices in quantification of protein abundance level to determine interindividual variability in DMET proteins by LC-MS/MS proteomics. Clin Pharmacol Ther. 2018;103:619–30.
Prasad B, Achour B, Artursson P, Hop CECA, Lai Y, Smith PC, et al. Toward a consensus on applying quantitative liquid chromatography-tandem mass spectrometry proteomics in translational pharmacology research: a white paper. Clin Pharmacol Ther. 2019;106:525–43.
Choi JM, Oh SJ, Lee SY, Im JH, Oh JM, Ryu CS, et al. HepG2 cells as an in vitro model for evaluation of cytochrome P450 induction by xenobiotics. Arch Pharm Res. 2015;38:691–704.
Acknowledgements
The study was partially financially supported by a grant from the National Natural Science Foundation of China (No. 81903701). We would like to acknowledge Dr. Shou-yan Wu and Prof. Li-kun Gong for their help in cell transfection experiments. Also, we appreciate all members in our laboratory for their kind help in daily work.
Author information
Authors and Affiliations
Contributions
YLW and ZTG designed and performed the experiments. YLW, DFZ, and XXD wrote the manuscript. YLW and ZDC performed the sample analysis and LC-MS/MS maintenance. YLW and YRX performed the isolation of rat/mouse primary hepatocytes. YLW, YRX, and XYG analyzed the RNA and protein samples of mouse hepatocytes.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. The authors alone are responsible for the content and writing of this manuscript.
Rights and permissions
About this article
Cite this article
Wu, Yl., Xue, Yr., Guo, Zt. et al. Furmonertinib (Alflutinib, AST2818) is a potential positive control drug comparable to rifampin for evaluation of CYP3A4 induction in sandwich-cultured primary human hepatocytes. Acta Pharmacol Sin 43, 747–756 (2022). https://doi.org/10.1038/s41401-021-00692-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00692-7