Abstract
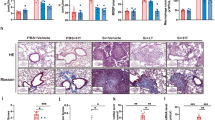
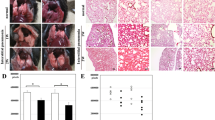
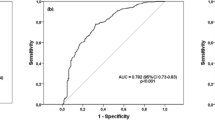
Silicosis is a global occupational disease characterized by lung dysfunction, pulmonary inflammation, and fibrosis, for which there is a lack of effective drugs. Pirfenidone has been shown to exert anti-inflammatory and anti-fibrotic properties in the lung. However, whether and how pirfenidone is effective against silicosis remains unknown. Here, we evaluated the efficacy of pirfenidone in the treatment of early and advanced silicosis in an experimental mouse model and explored its potential pharmacological mechanisms. We found that pirfenidone alleviated silica-induced lung dysfunction, secretion of inflammatory cytokines (TNF-α, IL-1β, IL-6) and deposition of fibrotic proteins (collagen I and fibronectin) in both early and advanced silicosis models. Moreover, we observed that both 100 and 200 mg/kg pirfenidone can effectively treat early-stage silicosis, while 400 mg/kg was recommended for advanced silicosis. Mechanistically, antibody array and bioinformatic analysis showed that the pathways related to IL-17 secretion, including JAK-STAT pathway, Th17 differentiation, and IL-17 pathway, might be involved in the treatment of silicosis by pirfenidone. Further in vivo experiments confirmed that pirfenidone reduced the production of IL-17A induced by silica exposure via inhibiting STAT3 phosphorylation. Neutralizing IL-17A by anti-IL-17A antibody improved lung function and reduced pulmonary inflammation and fibrosis in silicosis animals. Collectively, our study has demonstrated that pirfenidone effectively ameliorated silica-induced lung dysfunction, pulmonary inflammation and fibrosis in mouse models by inhibiting the secretion of IL-17A.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Leung CC, Yu IT, Chen W. Silicosis. Lancet. 2012;379:2008–18.
Barnes H, Goh N, Leong TL, Hoy R. Silica-associated lung disease: an old-world exposure in modern industries. Respirology. 2019;24:1165–75.
Lopes-Pacheco M, Bandeira E, Morales MM. Cell-based therapy for silicosis. Stem Cells Int. 2016;2016:50918–38.
Ruwanpura SM, Thomas BJ, Bardin PG. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Respir Cell Mol Biol. 2020;62:413–22.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9.
Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26:146.
George PM, Wells AU. Pirfenidone for the treatment of idiopathic pulmonary fibrosis. Expert Rev Clin Pharmacol. 2017;10:483–91.
Lopez-de LMD, Sanchez-Roque C, Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S, Macias-Barragan J, et al. Role and new insights of pirfenidone in fibrotic diseases. Int J Med Sci. 2015;12:840–7.
Cao Z, Song M, Liu Y, Pang J, Li Z, Qi X, et al. A novel pathophysiological classification of silicosis models provides some new insights into the progression of the disease. Ecotoxicol Environ Saf. 2020;202:1108–34.
Kurschus FC, Moos S. IL-17 for therapy. J Dermatol Sci. 2017;87:221–7.
Gurczynski SJ, Moore BB. IL-17 in the lung: the good, the bad, and the ugly. Am J Physiol Lung Cell Mol Physiol. 2018;314:L6–16.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76.
Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477–85.
Lo RS, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010;184:6367–77.
Pollard KM. Silica, silicosis, and autoimmunity. Front Immunol. 2016;7:97.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7.
Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:756–61.
Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2018;197:356–63.
Inomata M, Kamio K, Azuma A, Matsuda K, Kokuho N, Miura Y, et al. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis. Respir Res. 2014;15:16.
Li Y, Li H, Liu S, Pan P, Su X, Tan H, et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol. 2018;99:134–44.
Liu J, Shi G. Pirfenidone activates cannabinoid receptor 2 in a mouse model of bleomycin-induced pulmonary fibrosis. Exp Ther Med. 2019;18:4241–8.
Saleh MA, Antar SA, Hazem RM, El-Azab MF. Pirfenidone and vitamin D ameliorate cardiac fibrosis induced by doxorubicin in Ehrlich ascites carcinoma bearing mice: modulation of monocyte chemoattractant protein-1 and Jun N-terminal kinase-1 pathways. Pharmaceuticals 2020;13:11.
Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–99.
King EJ. Silicosis. Lect Sci Basis Med. 1952;2:108–38.
Lawrence M, Huber W, Pages H, Aboyoun P, Carlson M, Gentleman R, et al. Software for computing and annotating genomic ranges. PLoS Comput Biol. 2013;9:e1003118.
Omland O, Wurtz ET, Aasen TB, Blanc P, Brisman JB, Miller MR, et al. Occupational chronic obstructive pulmonary disease: a systematic literature review. Scand J Work Environ Health. 2014;40:19–35.
Camporeale A. Poli VIL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci. 2012;17:2306–26.
Guo J, Yang Z, Jia Q, Bo C, Shao H, Zhang Z. Pirfenidone inhibits epithelial-mesenchymal transition and pulmonary fibrosis in the rat silicosis model. Toxicol Lett. 2019;300:59–66.
Chen Y, Li C, Weng D, Song L, Tang W, Dai W, et al. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicol Appl Pharmacol. 2014;275:62–72.
Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–94.
Ahmed S, Misra DP, Agarwal V. Interleukin-17 pathways in systemic sclerosis-associated fibrosis. Rheumatol Int. 2019;39:1135–43.
Acknowledgements
This work was financially supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [grant number: 2018-12M-1-001] (to CW), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [grant number: 2018RC31001] (to CW) and the National Natural Science Foundation of China (91739107) (to JW).
Author information
Authors and Affiliations
Contributions
ZJC designed the project, performed experiments, analyzed data and drafted the manuscript. YL designed the project, performed experiments and analyzed data. ZZ, ZGL, MYS, XMQ, BCL, and XRZ contributed to the construction of silicosis models and helped collect experimental samples. JLP and ZFH performed bioinformatic analysis. PRY, HPD, JW, and CW helped design the project, commented on the manuscript and supervised all aspects of the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Cao, Zj., Liu, Y., Zhang, Z. et al. Pirfenidone ameliorates silica-induced lung inflammation and fibrosis in mice by inhibiting the secretion of interleukin-17A. Acta Pharmacol Sin 43, 908–918 (2022). https://doi.org/10.1038/s41401-021-00706-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00706-4
Keywords
This article is cited by
-
Association of Pirfenidone and its Cardioprotective effects in Pentylenetetrazole-induced kindling model of Epilepsy through the High mobility group Box-1/Toll-Like Receptor-4 pathway
Molecular Biology Reports (2026)
-
Pirfenidone targeted mechanisms for alleviating methotrexate-induced testiculopathy in Wistar rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Results from omic approaches in rat or mouse models exposed to inhaled crystalline silica: a systematic review
Particle and Fibre Toxicology (2024)
-
Crystalline silica-induced recruitment and immuno-imbalance of CD4+ tissue resident memory T cells promote silicosis progression
Communications Biology (2024)
-
Evaluation of prevention and treatment effects of fibroblast growth factor-21 in BLM-induced pulmonary fibrosis
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)