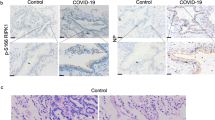
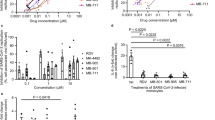
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can induce acute inflammatory response like acute lung inflammation (ALI) or acute respiratory distress syndrome, leading to severe progression and mortality. Therapeutics for treatment of SARS-CoV-2-triggered respiratory inflammation are urgent to be discovered. Our previous study shows that Salvianolic acid C potently inhibits SARS-CoV-2 infection. In this study, we investigated the antiviral effects of a Salvia miltiorrhiza compound, Danshensu, in vitro and in vivo, including the mechanism of S protein-mediated virus attachment and entry into target cells. In authentic and pseudo-typed virus assays in vitro, Danshensu displayed a potent antiviral activity against SARS-CoV-2 with EC50 of 0.97 μM, and potently inhibited the entry of SARS-CoV-2 S protein-pseudo-typed virus (SARS-CoV-2 S) into ACE2-overexpressed HEK-293T cells (IC50 = 0.31 μM) and Vero-E6 cell (IC50 = 4.97 μM). Mice received SARS-CoV-2 S via trachea to induce ALI, while the VSV-G treated mice served as controls. The mice were administered Danshensu (25, 50, 100 mg/kg, i.v., once) or Danshensu (25, 50, 100 mg·kg-1·d-1, oral administration, for 7 days) before SARS-CoV-2 S infection. We showed that SARS-CoV-2 S infection induced severe inflammatory cell infiltration, severely damaged lung tissue structure, highly expressed levels of inflammatory cytokines, and activated TLR4 and hyperphosphorylation of the NF-κB p65; the high expression of angiotensinogen (AGT) and low expression of ACE2 at the mRNA level in the lung tissue were also observed. Both oral and intravenous pretreatment with Danshensu dose-dependently alleviated the pathological alterations in mice infected with SARS-CoV-2 S. This study not only establishes a mouse model of pseudo-typed SARS-CoV-2 (SARS-CoV-2 S) induced ALI, but also demonstrates that Danshensu is a potential treatment for COVID-19 patients to inhibit the lung inflammatory response.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zheng L, Zhang L, Huang J, Nandakumar KS, Liu S, Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur J Med Chem. 2020;205:112687.
McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57:933–40.
Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–50.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Li YP, Ma Y, Wang N, Jin ZB. Eyes on coronavirus. Stem Cell Res. 2021;51:102200.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Acter T, Uddin N, Das J, Akhter A, Choudhury TR, Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci Total Environ. 2020;730:138996.
Peiris J, Chu C, Cheng V, Chan K, Hung I, Poon L, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72.
Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–15.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710.
Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res J Lab Clin Med. 2016;167:183–91.
Chan JF, Kok KH. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020; 9:221–36.
Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–23.
Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell host microbe. 2020;27:325–8.
Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4.
Zhang J, Zhang Q, Liu G, Zhang N. Therapeutic potentials and mechanisms of the Chinese traditional medicine Danshensu. Eur J Pharmacol. 2019;864:172710.
Bao XY, Zheng Q, Tong Q, Zhu PC, Zhuang Z, Zheng GQ, et al. Danshensu for myocardial ischemic injury: preclinical evidence and novel methodology of quality assessment tool. Front Pharmacol. 2018;9:1445.
Xu H, Liu W, Liu T, Su N, Guo C, Feng X, et al. Synergistic neuroprotective effects of Danshensu and hydroxysafflor yellow A on cerebral ischemia-reperfusion injury in rats. Oncotarget. 2017;8:115434–43.
Jiang L, Wang J, Ju J, Dai J. Salvianolic acid B and sodium tanshinone II A sulfonate prevent pulmonary fibrosis through anti-inflammatory and anti-fibrotic process. Eur J Pharmacol. 2020;883:173352.
Luo J, Zhang L, Zhang X, Long Y, Zou F, Yan C, et al. Protective effects and active ingredients of Salvia miltiorrhiza Bunge extracts on airway responsiveness, inflammation and remodeling in mice with ovalbumin-induced allergic asthma. Phytomedicine. 2019;52:168–77.
Yang C, Pan X, Xu X, Cheng C, Huang Y, Li L, et al. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal Transduct Target Ther. 2020; 5: 220.
Ye R, Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 2020;113:104350.
Chan CK, Tan LT, Andy SN, Kamarudin MNA, Goh BH, Kadir HA. Anti-neuroinflammatory activity of Elephantopus scaber L. via activation of Nrf2/HO-1 signaling and inhibition of p38 MAPK pathway in LPS-induced microglia BV-2 cells. Front Pharmacol. 2017;8:397.
Wang P, Zhang L, Liao Y, Du J, Xu M, Zhao W, et al. Effect of Intratracheal instillation of ZnO nanoparticles on acute lung inflammation induced by lipopolysaccharides in mice. Toxicol Sci. 2020;173:373–86.
Wang W, Wu L, Li Q, Zhang Z, Xu L, Lin C, et al. Madecassoside prevents acute liver failure in LPS/D-GalN-induced mice by inhibiting p38/NF-κB and activating Nrf2/HO-1 signaling. Biomed Pharmacother. 2018;103:1137–45.
Li L, Liu Y, Zhao X, Qi C, Zhang Y, Zhang Y, et al. Salvianic acid A sodium promotes the recovery of motor function after spinal cord injury in rats by reducing microglia inflammation through regulating MIP2/Vdac1/Ndufa12 signaling axis. Orthop Surg. 2020;12:1971–9.
Li Y, Chen H, Yang Y, Niu M, Wang J, Wu Y, et al. Danshen formula granule and salvianic acid A alleviate ethanol-induced neurotoxicity. J Nat Med. 2020;74:399–408.
Jia D, Zhang CZ, Qiu Y, Chen XF, Jia L, Chen AF, et al. Cardioprotective mechanisms of salvianic acid A sodium in rats with myocardial infarction based on proteome and transcriptome analysis. Acta Pharmacol Sin. 2019;40:1513–22.
Bourgonje A. R., Abdulle A. E., Timens W. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020; 251: 228-48.
Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9:680–6.
Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997;202:49–57.
Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–20.
Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–49.
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–46.
Han J, Lee JD, Bibbs L, Ulevitch RJA. MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11.
Seneviratne AN, Monaco C. Role of inflammatory cells and toll-like receptors in atherosclerosis. Curr Vasc Pharmacol. 2015;13:146–60.
Yang XL, Guo TK, Wang YH, Huang YH, Liu X, Wang XX, et al. Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int Immunopharmacol. 2012;12:408–14.
Wang S, Hibberd ML, Pettersson S, Lee YK. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS One. 2014;9:e97523.
Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34.
Enomoto N, Takei Y, Hirose M, Ikejima K, Miwa H, Kitamura T, Sato N. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization and TNF-alpha production. Gastroenterology. 2002;123:291–300.
Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71.
Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11.
Mattson MP, Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–54.
Lam FF, Yeung JH, Chan KM, Or PM. Relaxant effects of danshen aqueous extract and its constituent danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vasc Pharmacol. 2007;46:271–7.
Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020; 27:3209–25.
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020; 581: 221–4.
Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904.e9.
Wan Y., Shang J., Graham R., Baric R. S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94:e00127–20.
Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–8.
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–92.e6.
Yan R., Zhang Y. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020; 367:1444-8.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6.
Yilin Z, Yandong N, Faguang J. Role of angiotensin-converting enzyme (ACE) and ACE2 in a rat model of smoke inhalation induced acute respiratory distress syndrome. Burns. 2015;41:1468–77.
Hansen J, Baum A. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–4.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9.
Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–74.
Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, et al. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med. 2012;90:637–47.
Hemnes AR, Rathinasabapathy A, Austin EA, Brittain EL, Carrier EJ, Chen X, et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018;51:1702638.
Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care (Lond, Engl). 2017;21:234.
Wang J, Kaplan N, Wysocki J, Yang W, Lu K, Peng H, et al. The ACE2-deficient mouse: a model for a cytokine storm-driven inflammation. FASEB J. 2020;34:10505–15.
Tyagi SC, Singh M. Multi-organ damage by Covid-19: congestive (cardio-pulmonary) heart failure, and blood-heart barrier leakage. Mol Cell Biochem. 2021;476:1891–5.
Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci. 2014;126:461–9.
Acknowledgements
These authors gratefully acknowledge financial support from the Major Scientific and Technological Projects of Guangdong Province (2019B020202002) and Chinese Academy of Traditional Chinese Medicine (ZZ13-035-02, 2019XZZX-LG04) to SWL; Guangzhou Science and Technology Program (202008040001, 201803040006 to WX and 2020B111110001 to SWL); National Natural Science Foundation of China (82070083 to GDH).
Author information
Authors and Affiliations
Contributions
SWL contributed to the conception and design; WW, SSL, XFX, CY, XGN, SXY, XYP, WX, and GDH contributed to the acquisition of data or analysis and interpretation of data; WW, SSL and XFX drafted the paper; SWL and CW supervised the experiments. All authors read and approved the final version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Wang, W., Li, Ss., Xu, Xf. et al. Danshensu alleviates pseudo-typed SARS-CoV-2 induced mouse acute lung inflammation. Acta Pharmacol Sin 43, 771–780 (2022). https://doi.org/10.1038/s41401-021-00714-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00714-4
Keywords
This article is cited by
-
Self-sufficient biocatalytic cascade for the continuous synthesis of danshensu in flow
Applied Microbiology and Biotechnology (2025)
-
Exploring the potential mechanisms of Danshen against COVID-19 via network pharmacology analysis and molecular docking
Scientific Reports (2024)
-
Ethyl Lithospermate Reduces Lipopolysaccharide-Induced Inflammation through Inhibiting NF-κB and STAT3 Pathways in RAW 264.7 Cells and Zebrafish
Chinese Journal of Integrative Medicine (2023)
-
Small molecules in the treatment of COVID-19
Signal Transduction and Targeted Therapy (2022)