Abstract
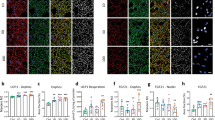
β-Adrenergic receptor (β-AR) overactivation is a major pathological factor associated with cardiac diseases and mediates cardiac inflammatory injury. Glibenclamide has shown anti-inflammatory effects in previous research. However, it is unclear whether and how glibenclamide can alleviate cardiac inflammatory injury induced by β-AR overactivation. In the present study, male C57BL/6J mice were treated with or without the β-AR agonist isoprenaline (ISO) with or without glibenclamide pretreatment. The results indicated that glibenclamide alleviated ISO-induced macrophage infiltration in the heart, as determined by Mac-3 staining. Consistent with this finding, glibenclamide also inhibited ISO-induced chemokines and proinflammatory cytokines expression in the heart. Moreover, glibenclamide inhibited ISO-induced cardiac fibrosis and dysfunction in mice. To reveal the protective mechanism of glibenclamide, the NLRP3 inflammasome was further analysed. ISO activated the NLRP3 inflammasome in both cardiomyocytes and mouse hearts, but this effect was alleviated by glibenclamide pretreatment. Furthermore, in cardiomyocytes, ISO increased the efflux of potassium and the generation of ROS, which are recognized as activators of the NLRP3 inflammasome. The ISO-induced increases in these processes were inhibited by glibenclamide pretreatment. Moreover, glibenclamide inhibited the cAMP/PKA signalling pathway, which is downstream of β-AR, by increasing phosphodiesterase activity in mouse hearts and cardiomyocytes. In conclusion, glibenclamide alleviates β-AR overactivation-induced cardiac inflammation by inhibiting the NLRP3 inflammasome. The underlying mechanism involves glibenclamide-mediated suppression of potassium efflux and ROS generation by inhibiting the cAMP/PKA pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Carter JR, Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol. 2015;5:119–46.
Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:133–44.
Gao R, Yang T, Xu W. Enemies or weapons in hands: investigational anti-diabetic drug glibenclamide and cancer risk. Expert Opin Investig Drugs. 2017;26:853–64.
Cui W, Zhang S, Cai Z, Hu X, Zhang R, Wang Y, et al. The antidiabetic agent glibenclamide protects airway hyperresponsiveness and inflammation in mice. Inflammation. 2015;38:835–45.
Carvalho AM, Novais FO, Paixao CS, de Oliveira CI, Machado P, Carvalho LP, et al. Glyburide, a NLRP3 inhibitor, decreases inflammatory response and is a candidate to reduce pathology in leishmania braziliensis infection. J Invest Dermatol. 2020;140:246–9.
Xiao H, Li H, Wang JJ, Zhang JS, Shen J, An XB, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon beta-adrenergic insult. Eur Heart J. 2018;39:60–9.
Shen J, Wu JM, Hu GM, Li MZ, Cong WW, Feng YN, et al. Membrane nanotubes facilitate the propagation of inflammatory injury in the heart upon overactivation of the beta-adrenergic receptor. Cell Death Dis. 2020;11:958.
Wang J, Chai J. Molecular actions of NLR immune receptors in plants and animals. Sci China Life Sci. 2020;63:1–14.
Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Asp Med. 2020;76:100889.
Zhang J, Xiao H, Shen J, Wang N, Zhang Y. Different roles of beta-arrestin and the PKA pathway in mitochondrial ROS production induced by acute beta-adrenergic receptor stimulation in neonatal mouse cardiomyocytes. Biochem Biophys Res Commun. 2017;489:393–8.
Cao N, Chen H, Bai Y, Yang X, Xu W, Hao W, et al. beta2-adrenergic receptor autoantibodies alleviated myocardial damage induced by beta1-adrenergic receptor autoantibodies in heart failure. Cardiovasc Res. 2018;114:1487–98.
Alemasi A, Cao N, An X, Wu J, Gu H, Yu H, et al. Exercise attenuates acute beta-adrenergic overactivation-induced cardiac fibrosis by modulating cytokines. J Cardiovasc Transl Res. 2019;12:528–38.
Xin JZ, Wu JM, Hu GM, Gu HJ, Feng YN, Wang SX, et al. alpha1-AR overactivation induces cardiac inflammation through NLRP3 inflammasome activation. Acta Pharmacol Sin. 2020;41:311–8.
Bezine M, Debbabi M, Nury T, Ben-Khalifa R, Samadi M, Cherkaoui-Malki M, et al. Evidence of K+ homeostasis disruption in cellular dysfunction triggered by 7-ketocholesterol, 24S-hydroxycholesterol, and tetracosanoic acid (C24:0) in 158N murine oligodendrocytes. Chem Phys Lipids. 2017;207:135–50.
Kumar M, Hsiao K, Vidugiriene J, Goueli SA. A bioluminescent-based, HTS-compatible assay to monitor G-protein-coupled receptor modulation of cellular cyclic AMP. Assay Drug Dev Technol. 2007;5:237–45.
Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215–29.
Zhang G, Lin X, Zhang S, Xiu H, Pan C, Cui W. A protective role of glibenclamide in inflammation-associated injury. Mediators Inflamm. 2017;2017:3578702.
Liao J, Kapadia VS, Brown LS, Cheong N, Longoria C, Mija D, et al. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun. 2015;6:8977.
Cai J, Lu S, Yao Z, Deng YP, Zhang LD, Yu JW, et al. Glibenclamide attenuates myocardial injury by lipopolysaccharides in streptozotocin-induced diabetic mice. Cardiovasc Diabetol. 2014;13:106.
Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care. 2017;40:706–14.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352: 837–53.
Cacciapuoti F, Spiezia R, Bianchi U, Lama D, D’Avino M, Varricchio M. Effectiveness of glibenclamide on myocardial ischemic ventricular arrhythmias in non-insulin-dependent diabetes mellitus. Am J Cardiol. 1991;67:843–7.
Leonard CE, Brensinger CM, Aquilante CL, Bilker WB, Boudreau DM, Deo R, et al. Comparative safety of sulfonylureas and the risk of sudden cardiac arrest and ventricular arrhythmia. Diabetes Care. 2018;41:713–22.
Jha JC, Ho F, Dan C, Jandeleit-Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci (Lond). 2018;132:1811–36.
Diwan V, Gobe G, Brown L. Glibenclamide improves kidney and heart structure and function in the adenine-diet model of chronic kidney disease. Pharmacol Res. 2014;79:104–10.
Hughes FJ, Vivar NP, Kennis JG, Pratt-Thomas JD, Lowe DW, Shaner BE, et al. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Ren Physiol. 2014;306:F299–F308.
He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–21.
Nawrath H, Blei I, Gegner R. Opposite effects of beta-adrenoceptor stimulation and 8-bromo-cyclic AMP on potassium efflux in mammalian heart muscle. Experientia 1980;36:72–4.
Nichols CG, Singh GK, Grange DK. KATP channels and cardiovascular disease: suddenly a syndrome. Circ Res. 2013;112:1059–72.
Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, et al. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–R1214.
Muller G, Wied S, Wetekam EM, Crecelius A, Unkelbach A, Punter J. Stimulation of glucose utilization in 3T3 adipocytes and rat diaphragm in vitro by the sulphonylureas, glimepiride and glibenclamide, is correlated with modulations of the cAMP regulatory cascade. Biochem Pharmacol. 1994;48:985–96.
Acknowledgements
This work was supported by the National Key R&D Program (grant number 2020YFC2004704 to Han Xiao), UMHS-PUHSC Joint Institute for Translational and Clinical Research (grant number BMU2019JI007 to You-yi Zhang), the Natural Science Foundation of China (grant numbers 81822003 and 81670205 to Han Xiao; 81830009 to You-yi Zhang), and the Beijing Municipal Natural Science Foundation (grant number 7191013 to Er-dan Dong), and the Key Clinical Projects of Peking University Third Hospital (grant number BYSYZD2019022 to Han Xiao).
Author information
Authors and Affiliations
Contributions
HX, YYZ and EDD conceived the project and designed the study. NC, JJW, JMW, WLX, RW, XDC, YNF and WWC performed the experiments. NC and JJW analysed the data. NC and JMW wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cao, N., Wang, Jj., Wu, Jm. et al. Glibenclamide alleviates β adrenergic receptor activation-induced cardiac inflammation. Acta Pharmacol Sin 43, 1243–1250 (2022). https://doi.org/10.1038/s41401-021-00734-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00734-0
Keywords
This article is cited by
-
Miglustat ameliorates isoproterenol-induced cardiac fibrosis via targeting UGCG
Molecular Medicine (2025)
-
Identification and experimental validation of mitochondrial and endoplasmic reticulum stress related gene in diabetic nephropathy
Scientific Reports (2025)
-
Glutaminolysis and α-ketoglutarate-stimulated KCa3.1 expression contribute to β-adrenoceptor activation-induced myocardial fibrosis in mice
Science China Life Sciences (2025)
-
P2X7 receptor is essential for ST36-attenuated cardiac fibrosis upon beta-adrenergic insult
Purinergic Signalling (2025)
-
Isoproterenol induces MD2 activation by β-AR-cAMP-PKA-ROS signalling axis in cardiomyocytes and macrophages drives inflammatory heart failure
Acta Pharmacologica Sinica (2024)