Abstract
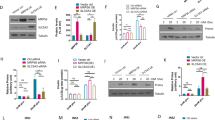
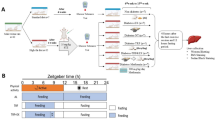
Diabetes is often associated with vitamin A disorders. All-trans retinoic acid (ATRA) is the main active constituent of vitamin A. We aimed to investigate whether ATRA influences diabetic progression and its mechanisms using both Goto-Kazizazi (GK) rats and INS-1 cells. Rat experiments demonstrated that ATRA treatment worsened diabetes symptoms, as evidenced by an increase in fasting blood glucose (FBG) levels and impairment of glucose homeostasis. Importantly, ATRA impaired glucose-stimulated insulin secretion (GSIS) and increased the expression of sterol regulatory element-binding protein 1c (SREBP-1c) and uncoupling protein 2 (UCP2) in the rat pancreas. Data from INS-1 cells also showed that ATRA upregulated SREBP-1c and UCP2 expression and impaired GSIS at 23 mM glucose. Srebp-1c or Ucp2 silencing attenuated GSIS impairment by reversing the ATRA-induced increase in UCP2 expression and decrease in ATP content. ATRA and the retinoid X receptor (RXR) agonists 9-cis RA and LG100268 induced the gene expression of Srebp-1c, which was almost completely abolished by the RXR antagonist HX531. RXRα-LBD luciferase reporter plasmid experiments also demonstrated that ATRA concentration-dependently activated RXRα, the EC50 of which was 1.37 μM, which was lower than the ATRA concentration in the pancreas of GK rats treated with a high dose of ATRA (approximately 3 μM), inferring that ATRA can upregulate Srebp-1c expression in the pancreas by activating RXR. In conclusion, ATRA impaired GSIS partly by activating the RXR/SREBP-1c/UCP2 pathway, thus worsening diabetic symptoms. The results highlight the roles of ATRA in diabetic progression and establish new strategies for diabetes treatment.

Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30.
Kane MA. Analysis, occurrence, and function of 9-cis-retinoic acid. Biochim Biophys Acta. 2012;1821:10–20.
Li R, Zhang R, Li Y, Zhu B, Chen W, Zhang Y, et al. A RARE of hepatic Gck promoter interacts with RARα, HNF4α and COUP-TFII that affect retinoic acid- and insulin-induced Gck expression. J Nutr Biochem. 2014;25:964–76.
Hurst RJM, Else KJ. Retinoic acid signalling in gastrointestinal parasite infections: lessons from mouse models. Parasite Immunol. 2012;34:351–9.
Zhang R, Wang Y, Li R, Chen G. Transcriptional factors mediating retinoic acid signals in the control of energy metabolism. Int J Mol Sci. 2015;16:14210–44.
Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–808.
Iqbal S, Naseem I. Role of vitamin A in type 2 diabetes mellitus biology: effects of intervention therapy in a deficient state. Nutrition. 2015;31:901–7.
Olsen T, Blomhoff R. Retinol, retinoic acid, and retinol-binding protein 4 are differentially associated with cardiovascular disease, type 2 diabetes, and obesity: an overview of human studies. Adv Nutr. 2020;11:644–66.
Zhang M, Liu C, Hu M, Zhang J, Xu P, Li F, et al. High-fat diet enhanced retinal dehydrogenase activity, but suppressed retinol dehydrogenase activity in liver of rats. J Pharm Sci. 2015;127:430–8.
Xu J, Zhang M, Zhang X, Yang H, Sun B, Wang Z, et al. Contribution of hepatic retinaldehyde dehydrogenase induction to impairment of glucose metabolism by high-fat-diet feeding in C57BL/6J mice. Basic Clin Pharmacol Toxicol. 2018;123:539–48.
Kim-Muller JY, Fan J, Kim YJR, Lee S-A, Ishida E, Blaner WS, et al. Aldehyde dehydrogenase 1a3 defines a subset of failing pancreatic β cells in diabetic mice. Nat Commun. 2016;7:12631.
Shimamura M, Karasawa H, Sakakibara S, Shinagawa A. Raldh3 expression in diabetic islets reciprocally regulates secretion of insulin and glucagon from pancreatic islets. Biochem Biophys Res Commun. 2010;401:79–84.
Koistinen HA, Remitz A, Gylling H, Miettinen TA, Koivisto VA, Ebeling P. Dyslipidemia and a reversible decrease in insulin sensitivity induced by therapy with 13-cis-retinoic acid. Diabetes Metab Res Rev. 2001;17:391–5.
Heliövaara MK, Remitz A, Reitamo S, Teppo AM, Karonen SL, Ebeling P. 13-cis-Retinoic acid therapy induces insulin resistance, regulates inflammatory parameters, and paradoxically increases serum adiponectin concentration. Metabolism. 2007;56:786–91.
Gu W, Hu S, He B, Qiu G, Ma J, Chen Z. Metabolites of acute promyelocytic leukemia cells participate in contributing to hypertriglyceridemia induced by all-trans retinoic acid. Leuk Res. 2009;33:592–4.
Krupková M, Janků M, Liska F, Sedová L, Kazdová L, Krenová D, et al. Pharmacogenetic model of retinoic acid-induced dyslipidemia and insulin resistance. Pharmacogenomics. 2009;10:1915–27.
Jeyakumar SM, Sheril A, Vajreswari A. Chronic vitamin A-enriched diet feeding induces body weight gain and adiposity in lean and glucose-intolerant obese rats of WNIN/GR-Ob strain. Exp Physiol. 2015;100:1352–61.
Ravnskjaer K, Boergesen M, Rubi B, Larsen JK, Nielsen T, Fridriksson J, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) potentiates, whereas PPARgamma attenuates, glucose-stimulated insulin secretion in pancreatic beta-cells. Endocrinology. 2005;146:3266–76.
Oström M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, et al. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3:e2841.
Matthews KA, Rhoten WB, Driscoll HK, Chertow BS. Vitamin A deficiency impairs fetal islet development and causes subsequent glucose intolerance in adult rats. J Nutr. 2004;134:1958–63.
Brun PJ, Grijalva A, Rausch R, Watson E, Yuen JJ, Das BC, et al. Retinoic acid receptor signaling is required to maintain glucose-stimulated insulin secretion and β-cell mass. FASEB J. 2015;29:671–83.
Driscoll JE, Seachord CL, Lupisella JA, Darveau RP, Reczek PR. Ligand-induced conformational changes in the human retinoic acid receptor detected using monoclonal antibodies. J Biol Chem. 1996;271:22969–75.
McCarroll JA, Phillips PA, Santucci N, Pirola RC, Wilson JS, Apte MV. Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89.
Kane MA, Folias AE, Pingitore A, Perri M, Obrochta KM, Krois CR, et al. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2010;107:21884–9.
Chertow BS, Driscoll HK, Goking NQ, Primerano D, Cordle MB, Matthews KA. Retinoid-X receptors and the effects of 9-cis-retinoic acid on insulin secretion from RINm5F cells. Metabolism. 1997;46:656–60.
Miyazaki S, Taniguchi H, Moritoh Y, Tashiro F, Yamamoto T, Yamato E, et al. Nuclear hormone retinoid X receptor (RXR) negatively regulates the glucose-stimulated insulin secretion of pancreatic ß-cells. Diabetes. 2010;59:2854–61.
Abd Eldaim MA, Matsuoka S, Okamatsu-Ogura Y, Kamikawa A, Ahmed MM, Terao A, et al. Retinoic acid modulates lipid accumulation glucose concentration dependently through inverse regulation of SREBP-1 expression in 3T3L1 adipocytes. Genes Cells. 2017;22:568–82.
Li Y, Zhang Y, Li R, Chen W, Howell M, Zhang R, et al. The hepatic Raldh1 expression is elevated in Zucker fatty rats and its over-expression introduced the retinal-induced Srebp-1c expression in INS-1 cells. PLoS One. 2012;7:e45210.
Li R, Chen W, Li Y, Zhang Y, Chen G. Retinoids synergized with insulin to induce Srebp-1c expression and activated its promoter via the two liver X receptor binding sites that mediate insulin action. Biochem Biophys Res Commun. 2011;406:268–72.
Patanè G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes. 2002;51:2749–56.
Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–10.
Takahashi A, Motomura K, Kato T, Yoshikawa T, Nakagawa Y, Yahagi N, et al. Transgenic mice overexpressing nuclear SREBP-1c in pancreatic beta-cells. Diabetes. 2005;54:492–9.
Kato T, Shimano H, Yamamoto T, Ishikawa M, Kumadaki S, Matsuzaka T, et al. Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes. 2008;57:2382–92.
Shimano H, Amemiya-Kudo M, Takahashi A, Kato T, Ishikawa M, Yamada N. Sterol regulatory element-binding protein-1c and pancreatic beta-cell dysfunction. Diabetes Obes Metab. 2007;9:133–9. Suppl 2
Rao J, Zhang C, Wang P, Lu L, Zhang F. All-trans retinoic acid alleviates hepatic ischemia/reperfusion injury by enhancing manganese superoxide dismutase in rats. Biol Pharm Bull. 2010;33:869–75.
Ewees MG, Abdelghany TM, Abdel-Aziz AAH, Abdel-Bakky MS. All-trans retinoic acid mitigates methotrexate-induced liver injury in rats; relevance of retinoic acid signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:931–8.
Miwa H. High-performance liquid chromatographic determination of mono-, poly- and hydroxycarboxylic acids in foods and beverages as their 2-nitrophenylhydrazides. J Chromatogr A. 2000;881:365–85.
Yang YM, Huang DY, Liu GF, Zhong JC, Du K, Li YF, et al. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on vitamin A metabolism in mice. J Biochem Mol Toxicol. 2005;19:327–35.
Quan X, Zhang L, Li Y, Liang C. TCF2 attenuates FFA-induced damage in islet β-cells by regulating production of insulin and ROS. Int J Mol Sci. 2014;15:13317–32.
Li J, Liu X, Ran X, Chen J, Li X, Wu W, et al. Sterol regulatory element-binding protein-1c knockdown protected INS-1E cells from lipotoxicity. Diabetes Obes Metab. 2010;12:35–46.
Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, et al. Role of uncoupling protein-2 up-regulation and triglyceride accumulation in impaired glucose-stimulated insulin secretion in a beta-cell lipotoxicity model overexpressing sterol regulatory element-binding protein-1c. Endocrinology. 2004;145:3566–77.
Diraison F, Parton L, Ferré P, Foufelle F, Briscoe CP, Leclerc I, et al. Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR). Biochem J. 2004;378:769–78.
Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–55.
Tavridou A, Unwin NC, Laker MF, White M, Alberti KG. Serum concentrations of vitamins A and E in impaired glucose tolerance. Clin Chim Acta. 1997;266:129–40.
Kim M, Jee SH, Kim M, Yoo HJ, Kang M, Kim J, et al. Serum vitamin A-related metabolite levels are associated with incidence of type 2 diabetes. Diabetes Metab. 2017;43:287–91.
Chien CY, Yuan TA, Cho CHH, Chang FP, Mao WY, Wu RR, et al. All-trans retinoic acid ameliorates glycemic control in diabetic mice via modulating pancreatic islet production of vascular endothelial growth factor-A. Biochem Biophys Res Commun. 2016;477:874–80.
Eltony SA, Elmottaleb NA, Gomaa AM, Anwar MM, El-Metwally TH. Effect of all-trans retinoic acid on the pancreas of streptozotocin-induced diabetic rat. Anat Rec (Hoboken). 2016;299:334–51.
Wang Y, Zhong YJ, Wang YY, Xing J, Wang ZM. All-trans retinoic acid prevents the development of type 1 diabetes by affecting the levels of interferon gamma and interleukin 4 in streptozotocin-induced murine diabetes model. Genet Mol Res. 2016;15. https://doi.org/10.4238/gmr.15017522.
Blaner WS. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. 2019;197:153–78.
Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–200.
Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101:1044–54.
Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–96.
Chertow BS, Goking NQ, Driscoll HK, Primerano DA, Matthews KA. Effects of all-trans-retinoic acid (ATRA) and retinoic acid receptor (RAR) expression on secretion, growth, and apoptosis of insulin-secreting RINm5F cells. Pancreas. 1997;15:122–31.
Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–76.
Kamei Y, Miura S, Suganami T, Akaike F, Kanai S, Sugita S, et al. Regulation of SREBP1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology. 2008;149:2293–305.
Kiefer FW, Vernochet C, O’Brien P, Spoerl S, Brown JD, Nallamshetty S, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012;18:918–25.
Lancet JE, Moseley AB, Coutre SE, DeAngelo DJ, Othus M, Tallman MS. et al. A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535). Blood Adv. 2020;4:1683–9.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81673505, 81872930, and 82073922), “333” Project of Jiangsu Province (BRA2020287).
Author information
Authors and Affiliations
Contributions
HYY, ML, LL, and XDL designed the research. ML, HYY, YS, LZ, MMJ, TXJ, LY, and PHL completed the experiments. ML analyzed the data. HYY, ML, LL, and XDL wrote the paper. All authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yang, Hy., Liu, M., Sheng, Y. et al. All-trans retinoic acid impairs glucose-stimulated insulin secretion by activating the RXR/SREBP-1c/UCP2 pathway. Acta Pharmacol Sin 43, 1441–1452 (2022). https://doi.org/10.1038/s41401-021-00740-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00740-2
Keywords
This article is cited by
-
Ferroptosis in the pathogenesis of diabetic cardiomyopathy: mechanisms and therapeutic potential
Cardiovascular Diabetology (2025)
-
Clozapine impaired glucose-stimulated insulin secretion partly by increasing plasma 5-HT levels due to the inhibition of OCT1-mediated hepatic 5-HT uptake in mice
Acta Pharmacologica Sinica (2025)
-
GLP-1 receptor agonist protects glucose-stimulated insulin secretion in pancreatic β-cells against lipotoxicity via PPARδ/UCP2 pathway
Cellular and Molecular Life Sciences (2025)
-
Mitochondrial uncoupling protein 2: a central player in pancreatic disease pathophysiology
Molecular Medicine (2024)