Abstract
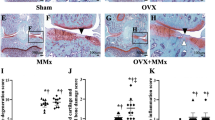
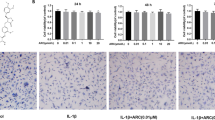
Osteoarthritis (OA) is the most common arthritis with a rapidly increasing prevalence. Disease progression is irreversible, and there is no curative therapy available. During OA onset, abnormal mechanical loading leads to excessive osteoclastogenesis and bone resorption in subchondral bone, causing a rapid subchondral bone turnover, cyst formation, sclerosis, and finally, articular cartilage degeneration. Moreover, osteoclast-mediated angiogenesis and sensory innervation in subchondral bone result in abnormal vascularization and OA pain. The traditional Chinese medicine Panax notoginseng (PN; Sanqi) has long been used in treatment of bone diseases including osteoporosis, bone fracture, and OA. In this study we established two-dimensional/bone marrow mononuclear cell/cell membrane chromatography/time of flight mass spectrometry (2D/BMMC/CMC/TOFMS) technique and discovered that diterbutyl phthalate (DP) was the active constituent in PN inhibiting osteoclastogenesis. Then we explored the therapeutic effect of DP in an OA mouse model with anterior cruciate ligament transaction (ACLT). After ACLT was conducted, the mice received DP (5 mg·kg–1·d–1, ip) for 8 weeks. Whole knee joint tissues of the right limb were harvested at weeks 2, 4, and 8 for analysis. We showed that DP administration impeded overactivated osteoclastogenesis in subchondral bone and ameliorated articular cartilage deterioration. DP administration blunted aberrant H-type vessel formation in subchondral bone marrow and alleviated OA pain assessed in Von Frey test and thermal plantar test. In RANKL-induced RAW264.7 cells in vitro, DP (20 μM) retarded osteoclastogenesis by suppressing osteoclast fusion through inhibition of the ERK/c-fos/NFATc1 pathway. DP treatment also downregulated the expression of dendritic cell-specific transmembrane protein (DC-STAMP) and d2 isoform of the vacuolar (H+) ATPase V0 domain (Atp6v0d2) in the cells. In conclusion, we demonstrate that DP prevents OA progression by inhibiting abnormal osteoclastogenesis and associated angiogenesis and neurogenesis in subchondral bone.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019:393:1745–59.
Zhang Z, Huang C, Jiang Q, Zheng Y, Liu Y, Liu S, et al. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Ann Transl Med. 2020;8:1213.
Tang X, Wang S, Zhan S, Niu J, Tao K, Zhang Y, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68:648–53.
Koff RS, Dart RC. Osteoarthritis of the knee. N Engl J Med. 2006;354:2508–9. author reply 9
Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12:632–44.
Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16:673–88.
Sun MM, Beier F, Ratneswaran A. Nuclear receptors as potential drug targets in osteoarthritis. Curr Opin Pharmacol. 2018;40:81–6.
Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2020;80:413–22.
Goldring SR. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:249–58.
Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–73.
Sharma AR, Jagga S, Lee SS, Nam JS. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14:19805–30.
Deveza LA, Loeser RF. Is osteoarthritis one disease or a collection of many? Rheumatology. 2018;57:iv34–iv42.
Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–206.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41.
Tateiwa D, Yoshikawa H, Kaito T. Cartilage and bone destruction in arthritis: pathogenesis and treatment strategy: a literature review. Cells. 2019;8:818.
Strassle BW, Mark L, Leventhal L, Piesla MJ, Jian Li X, Kennedy JD, et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthr Cartil. 2010;18:1319–28.
Bultink IE, Lems WF. Osteoarthritis and osteoporosis: what is the overlap? Curr Rheumatol Rep. 2013;15:328.
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–8.
Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12.
Zhu S, Zhu J, Zhen G, Hu Y, An S, Li Y, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129:1076–93.
Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66:1423–8.
Ni S, Ling Z, Wang X, Cao Y, Wu T, Deng R, et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat Commun. 2019;10:5643.
Karsdal MA, Bay-Jensen AC, Lories RJ, Abramson S, Spector T, Pastoureau P, et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann Rheum Dis. 2014;73:336–48.
Peng LH, Ko CH, Siu SW, Koon CM, Yue GL, Cheng WH, et al. In vitro & in vivo assessment of a herbal formula used topically for bone fracture treatment. J Ethnopharmacol. 2010;131:282–9.
Zhou X, Razmovski-Naumovski V, Chang D, Li C, Kam A, Low M, et al. Synergistic effects of Danshen (Salvia Miltiorrhiza Radix et Rhizoma) and Sanqi (Notoginseng Radix et Rhizoma) combination in inhibiting inflammation mediators in RAW264.7 cells. BioMed Res Int. 2016;2016:5758195.
Meng L, Huang Q, Li X, Liang P, Li Y, Huang X, et al. Genes induced by Panax notoginseng in a rodent model of ischemia-reperfusion injury. J Immunol Res. 2020;2020:8873261.
Wenxi D, Shufang D, Xiaoling Y, Liming Y. Panax notoginseng saponins suppress radiation-induced osteoporosis by regulating bone formation and resorption. Phytomedicine. 2015;22:813–9.
Chang SH, Sung HC, Choi Y, Ko SY, Lee BE, Baek DH, et al. Suppressive effect of AIF, a water extract from three herbs, on collagen-induced arthritis in mice. Int Immunopharmacol. 2005;5:1365–72.
Park SH, Kim SK, Shin IH, Kim HG, Choe JY. Effects of AIF on knee osteoarthritis patients: double-blind, randomized placebo-controlled study. Korean J Physiol Pharmacol. 2009;13:33–7.
Chen X, Cao Y, Zhang H, Zhu Z, Liu M, Liu H, et al. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Acontium carmichaeli. Anal Chem. 2014;86:4748–57.
Gu Y, Chen X, Wang R, Wang S, Wang X, Zheng L, et al. Comparative two-dimensional HepG2 and L02/ cell membrane chromatography/C18/time-of-flight mass spectrometry for screening selective anti-hepatoma components from Scutellariae Radix. J Pharm Biomed Anal. 2019;164:550–6.
Gu Y, Chen X, Wang Y, Liu Y, Zheng L, Li X, et al. Development of 3-mercaptopropyltrimethoxysilane (MPTS)-modified bone marrow mononuclear cell membrane chromatography for screening anti-osteoporosis components from Scutellariae Radix. Acta Pharm Sin B. 2020;10:1856–65.
Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr Cartil. 2007;15:1061–9.
Khorasani MS, Diko S, Hsia AW, Anderson MJ, Genetos DC, Haudenschild DR, et al. Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice. Arthritis Res Ther. 2015;17:30.
Zhu S, Chen K, Lan Y, Zhang N, Jiang R, Hu J. Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone. 2013;53:340–9.
Siebelt M, Waarsing JH, Groen HC, Müller C, Koelewijn SJ, de Blois E, et al. Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone. 2014;66:163–70.
Yeon JT, Kim KJ, Choi SW, Moon SH, Park YS, Ryu BJ, et al. Anti-osteoclastogenic activity of praeruptorin A via inhibition of p38/Akt-c-Fos-NFATc1 signaling and PLCγ-independent Ca2+ oscillation. PloS One. 2014;9:e88974.
Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75:1714–21.
Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18:S17–23.
Wei JL, Fu W, Ding YJ, Hettinghouse A, Lendhey M, Schwarzkopf R, et al. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis Res Ther. 2017;19:280.
Austin PJ, Wu A, Moalem-Taylor G. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp. 2012: 3393.
Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88.
Ahmad Dar A, Sangwan PL, Kumar A. Chromatography: an important tool for drug discovery. J Sep Sci. 2020;43:105–19.
Ding M, Danielsen CC, Hvid I. The effects of bone remodeling inhibition by alendronate on three-dimensional microarchitecture of subchondral bone tissues in guinea pig primary osteoarthrosis. Calcif Tissue Int. 2008;82:77–86.
Wang Y, Xu J, Zhang X, Wang C, Huang Y, Dai K, et al. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017;8:e2715.
Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315.
Li B, Yu F, Wu F, Wang K, Lou F, Zhang D, et al. Visual osteoclast fusion via a fluorescence method. Sci Rep. 2018;8:10184.
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–51.
Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–9.
Nakamura I, Takahashi N, Jimi E, Udagawa N, Suda T. Regulation of osteoclast function. Mod Rheumatol. 2012;22:167–77.
Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol. 2008;22:176–85.
Lee MS, Kim HS, Yeon JT, Choi SW, Chun CH, Kwak HB, et al. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J Immunol. 1950;2009:3390–9.
Müller G, Storz P, Bourteele S, Döppler H, Pfizenmaier K, Mischak H, et al. Regulation of Raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J. 1998;17:732–42.
Yan J, Zuo G, Sherchan P, Huang L, Ocak U, Xu W, et al. CCR1 activation promotes neuroinflammation through CCR1/TPR1/ERK1/2 signaling pathway after intracerebral hemorrhage in mice. Neurotherapeutics. 2020;17:1170–83.
Yang A, Suh WI, Kang NK, Lee B, Chang YK. MAPK/ERK and JNK pathways regulate lipid synthesis and cell growth of Chlamydomonas reinhardtii under osmotic stress, respectively. Sci Rep. 2018;8:13857.
Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9.
Zhen G, Cao X. Targeting TGFbeta signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014;35:227–36.
Li Y, Mu W, Xu B, Ren J, Wahafu T, Wuermanbieke S, et al. Artesunate, an anti-malaria agent, attenuates experimental osteoarthritis by inhibiting bone resorption and CD31(hi)Emcn(hi) vessel formation in subchondral bone. Front Pharmacol. 2019;10:685.
Cui Z, Xu C, Li X, Song J, Yu B. Treatment with recombinant lubricin attenuates osteoarthritis by positive feedback loop between articular cartilage and subchondral bone in ovariectomized rats. Bone. 2015;74:37–47.
Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4:221–9.
Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis. 2011;70:2011–21.
Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8.
Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–8.
Aso K, Shahtaheri SM, Hill R, Wilson D, McWilliams DF, Nwosu LN, et al. Contribution of nerves within osteochondral channels to osteoarthritis knee pain in humans and rats. Osteoarthr Cartil. 2020;28:1245–54.
Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol. 2015;80:965–78.
Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–22.
Pereira M, Petretto E, Gordon S, Bassett JHD, Williams GR, Behmoaras J. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci. 2018;131:jcs216267.
Kodama J, Kaito T. Osteoclast multinucleation: review of current literature. Int J Mol Sci. 2020;21:5685.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–8.
Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–37.
Li X, Wang L, Huang B, Gu Y, Luo Y, Zhi X, et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci Adv. 2020;6:eabb7135.
Acknowledgements
This work was supported by the National Key Research and Development Plan (2018YFC2001500); National Natural Science Foundation of China (NSFC) Key Research Program in Aging (91749204); National Natural Science Foundation of China (81771491, 81871099, 81972254); and Shanghai Rising-Star Program (21QA1412000). We thank the Experimental Center of Changhai Hospital for providing experimental instruments and reagents. The technique of 2D/BMMC/CMC/TOFMS is supported by the School of Pharmacy of Naval Medical University. We appreciate the work of Dr Yan-qiu Gu for screening DP from PN.
Author information
Authors and Affiliations
Contributions
CF, XC, and JCS designed the research. CF, JWG, YJW, XQL, and HZ performed the experiments. JC, YH, and YYJ analyzed the data. XC and JCS interpreted the data. CF wrote the original manuscript. XC reviewed and edited the manuscript. CF and XC finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Fang, C., Guo, Jw., Wang, Yj. et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol Sin 43, 1299–1310 (2022). https://doi.org/10.1038/s41401-021-00747-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00747-9
Keywords
This article is cited by
-
Repurposing metformin for treating osteoarthritis via leveraging Nrf2 signaling
Scientific Reports (2026)
-
Trachelogenin alleviates osteoarthritis by inhibiting osteoclastogenesis and enhancing chondrocyte survival
Chinese Medicine (2024)
-
Effect of umbilical cord blood-mononuclear cells on knee osteoarthritis in rabbits
Journal of Orthopaedic Surgery and Research (2024)