Abstract
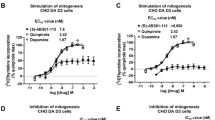
SLL-039 (N-cyclopropylmethyl-7α−4′-(N’-benzoyl) amino-phenyl-6,14-endoethano-tetrahydronorthebaine) and SLL-1206 (N-cyclopropylmethyl-7α−3′-(p-methoxybenzyl) amino-phenyl-6,14-endoethano-tetrahydronorthebaine) are two 4,5-epoxymorphinan-based high selective κ receptor agonists that we recently discovered. In the present study we characterized their pharmacological properties in comparison with arylacetamide-based typical κ agonist U50,488H. We showed that both SLL-039 and SLL-1206 produced potent and long-lasting antinociceptive actions in three different rodent models of pain via activation of κ opioid receptor. In hot-plate assay, the antinociceptive potency of SLL-039 and SLL-1206 increased about 11-and 17.3-fold compared to U50,488H and morphine, respectively, with ED50 values of 0.4 mg/kg. Following repeated administration, SLL-1206, SLL-039, and U50,488H all developed analgesic tolerance tested in hot-plate assay. U50,488H and SLL-039 produced antipruritic effects in a dose-dependent manner, whereas SLL-1206 displayed some antipruritic effects only at very low doses. In addition, SLL-1206 was capable of decreasing morphine-induced physical dependence. More importantly, SLL-039 and SLL-1206 at effective analgesic doses did not cause sedation and conditioned place aversion (CPA), whereas U50,488H did. In comparison with SLL-039, SLL-1206 caused similar antinociceptive responses, but fewer sedation and CPA. In conclusion, our results suggest that SLL-039 and SLL-1206 have potential to be developed as novel analgesic agents, and 4,5-expoxymorphinan scaffold is an attractive structure for the development of selective κ agonists with fewer side effects.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Stevens CW, Brasel CM, Mohan S. Cloning and bioinformatics of amphibian mu, delta, kappa, and nociceptin opioid receptors expressed in brain tissue: evidence for opioid receptor divergence in mammals. Neurosci Lett. 2007;419:189–94.
Rodriguez RE. Morphine and microRNA activity: is there a relation with addiction? Front Genet. 2012;3:223.
Abushanab D, Alsoukhni O, AbouNahia F, Al-Badriyeh D. Clinical and economic analysis of morphine versus fentanyl in managing ventilated neonates with respiratory distress syndrome in the intensive care setting. Clin Ther. 2019;41:714–27 e8.
Kiyatkin EA. Respiratory depression and brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin, and fentanyl. Neuropharmacology. 2019;151:219–26.
Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387–96.
Tao YM, Li QL, Zhang CF, Xu XJ, Chen J, Ju YW, et al. LPK-26, a novel kappa-opioid receptor agonist with potent antinociceptive effects and low dependence potential. Eur J Pharmacol. 2008;584:306–11.
Wang YJ, Tao YM, Li FY, Wang YH, Xu XJ, Chen J, et al. Pharmacological characterization of ATPM [(-)−3-aminothiazolo[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride], a novel mixed kappa-agonist and mu-agonist/-antagonist that attenuates morphine antinociceptive tolerance and heroin self-administration behavior. J Pharmacol Exp Ther. 2009;329:306–13.
Sun JF, Wang YH, Chai JR, Li FY, Hang A, Lu G, et al. Pharmacological characterization and therapeutic potential for the treatment of opioid abuse with ATPM-ET, an N-ethyl substituted aminothiazolomorphinan with kappa agonist and mu agonist/antagonist activity. Eur J Pharmacol. 2014;740:455–63.
Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL. Assessment of the kappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacology. 2014;231:2751–8.
Heinsbroek JA, Furbish AB, Peters J. A single, extinction-based treatment with a kappa opioid receptor agonist elicits a long-term reduction in cocaine relapse. Neuropsychopharmacology. 2018;43:1492–7.
Lahti RA, VonVoigtlander PF, Barsuhn C. Properties of a selective kappa agonist. U-50,488H Life Sci. 1982;31:2257–60.
Vonvoigtlander PF, Lahti RA, Ludens JH. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224:7–12.
Pande AC, Pyke RE, Greiner M, Wideman GL, Benjamin R, Pierce MW. Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clin Neuropharmacol. 1996;19:451–6.
Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology. 2001;157:151–62.
Vonvoigtlander PF, Lewis RA. Analgesic and mechanistic evaluation of spiradoline, a potent kappa opioid. J Pharmacol Exp Ther. 1988;246:259–62.
Rimoy GH, Wright DM, Bhaskar NK, Rubin PC. The cardiovascular and central nervous system effects in the human of U-62066E. A selective opioid receptor agonist. Eur J Clin Pharmacol. 1994;46:203–7.
Paton KF, Biggerstaff A, Kaska S, Crowley RS, La Flamme AC, Prisinzano TE, et al. Evaluation of biased and balanced salvinorin A analogs in preclinical models of pain. Front Neurosci. 2020;14:765.
Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009;94:244–9.
Morani AS, Schenk S, Prisinzano TE, Kivell BM. A single injection of a novel κ opioid receptor agonist salvinorin A attenuates the expression of cocaine-induced behavioral sensitization in rats. Behav Pharmacol. 2012;23:162–70.
Taylor GT, Manzella F. Kappa opioids, salvinorin A and major depressive disorder. Curr Neuropharmacol. 2016;14:165–76.
Endoh T, Tajima A, Izumimoto N, Suzuki T, Saitoh A, Suzuki T, et al. TRK-820, a selective kappa-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn J Pharmacol. 2001;85:282–90.
Nakao K, Hasebe K, Yoshikawa S, Ikeda K, Hirakata M, Miyamoto Y, et al. Pharmacological effects of nalfurafine hydrochloride, a kappa-opioid receptor agonist. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010;30(5-6):185-91.
Suzuki T, Izumimoto N, Takezawa Y, Fujimura M, Togashi Y, Nagase H, et al. Effect of repeated administration of TRK-820, a kappa-opioid receptor agonist, on tolerance to its antinociceptive and sedative actions. Brain Res. 2004;995:167–75.
Endoh T, Matsuura H, Tajima A, Izumimoto N, Tajima C, Suzuki T, et al. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999;65:1685–94.
Cao D, Huang P, Chiu YT, Chen C, Wang H, Li M, et al. Comparison of pharmacological properties between the kappa opioid receptor agonist nalfurafine and 42B, its 3-dehydroxy analogue: disconnect between in vitro agonist bias and in vivo pharmacological effects. ACS Chem Neurosci. 2020;11:3036–50.
Kozono H, Yoshitani H, Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch(®) capsules 2.5 μg) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9–24.
Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, et al. Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–83.
Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25:1251–7.
Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–7.
Liu JJ, Chiu YT, DiMattio KM, Chen C, Huang P, Gentile TA, et al. Phosphoproteomic approach for agonist-specific signaling in mouse brains: mTOR pathway is involved in κ opioid aversion. Neuropsychopharmacology. 2019;44:939–49.
Li W, Long JD, Qian YY, Long Y, Xu XJ, Wang YJ, et al. The Pharmacological heterogeneity of nepenthone analogs in conferring highly selective and potent κ-opioid agonistic activities. ACS Chem Neurosci. 2017;8:766–76.
Liu X, Jiang S, Kong L, Ye R, Xiao L, Xu X, et al. Exploration of the SAR connection between morphinan- and arylacetamide-based κ opioid receptor (κOR) agonists using the strategy of bridging. ACS Chem Neurosci. 2021;12:1018–30.
Sun HJ, Wang YH, Yuan CM, Kong LH, Xu XJ, Wang YJ, et al. 7β-Methyl substituent is a structural locus associated with activity cliff for nepenthone analogues. Bioorg Med Chem. 2018;26:4254–63.
Xiao L, Wang Y, Zhang M, Wu W, Kong L, Ma Y, et al. Discovery of a highly selective and potent κ opioid receptor agonist from N-cyclopropylmethyl-7α-phenyl-6,14-endoethanotetrahydronorthebaines with reduced central nervous system (CNS) side effects navigated by the message-address concept. J Med Chem. 2019;62:11054–70.
Li W, Tang Y, Zheng YL, Qiu ZB. Molecular modeling and 3D-QSAR studies of indolomorphinan derivatives as kappa opioid antagonists. Bioorg Med Chem. 2006;14:601–10.
Qi H, Wei LI, Qiu Y, Cui YY, Ma J, Gao XL, et al. Synthesis and evaluation of κ-opioid receptor agonistic activity and antinociceptive effect of novel morphine analogues, 7α-phenyl-6α,14α-endo-etheno-tetrahydrothebaine with substituted o-, m- and p-amino group. Med Chem Res. 2011;20:1364–70.
Zhang LS, Wang J, Chen JC, Tao YM, Wang YH, Xu XJ, et al. Novel kappa-opioid receptor agonist MB-1C-OH produces potent analgesia with less depression and sedation. Acta Pharmacol Sin. 2015;36:565–71.
Lu YC, Wang YJ, Lu B, Chen M, Zheng P, Liu JG. ACC to dorsal medial striatum inputs modulate histaminergic itch sensation. J Neurosci. 2018;38:3823–39.
Wang YH, Chai JR, Xu XJ, Ye RF, Zan GY, Liu GYK, et al. Pharmacological characterization of dezocine, a potent analgesic acting as a kappa partial agonist and mu partial agonist. Sci Rep. 2018;8:14087.
Liu JJ, Sharma K, Zangrandi L, Chen C, Humphrey SJ, Chiu YT, et al. In vivo brain GPCR signaling elucidated by phosphoproteomics. Science. 2018;360:eaao4927.
Inan S, Cowan A. Antipruritic effects of kappa opioid receptor agonists: evidence from rodents to humans. Handb Exp Pharmacol. 2020. https://doi.org/10.1007/164_2020_420.
Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, et al. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–64.
Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35:12917–31.
Kivell BM, Ewald AW, Prisinzano TE. Salvinorin A analogs and other κ-opioid receptor compounds as treatments for cocaine abuse. Adv Pharmacol. 2014;69:481–511.
Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–35.
Zhou JJ, Bian JS, Pei JM, Wu S, Li HY, Wong TM. Role of protein kinase C-epsilon in the development of kappa-opioid receptor tolerance to U50,488H in rat ventricular myocytes. Br J Pharmacol. 2002;135:1675–84.
He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–82.
Devine DP, Leone P, Wise RA. Mesolimbic dopamine neurotransmission is increased by administration of mu-opioid receptor antagonists. Eur J Pharmacol. 1993;243:55–64.
White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, et al. The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109.
Acknowledgements
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDA12040319 to JGL) and National Natural Science Foundation of China (Grant 81773710 to YJW and 82030112 to JGL), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (Grant 2017334 to YJW).
Author information
Authors and Affiliations
Contributions
JGL, YJW, WL, and LMS designed the experiments. YYW and YM performed the experiments with the assistance of SYY, JRC, and JC. LHK and XL synthesized compounds. YYW, YM, and YJW performed the statistical data analysis. YYW and YJW written this manuscript. JGL, WL, and LMS revised this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Wei, Yy., Ma, Y., Yao, Sy. et al. Novel selective κ agonists SLL-039 and SLL-1206 produce potent antinociception with fewer sedation and aversion. Acta Pharmacol Sin 43, 1372–1382 (2022). https://doi.org/10.1038/s41401-021-00761-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00761-x