Abstract
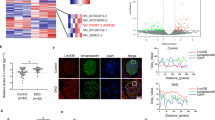
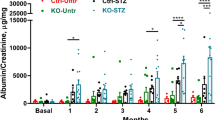
Panax notoginseng, a traditional Chinese medicine, exerts beneficial effect on diabetic kidney disease (DKD), but its mechanism is not well clarified. In this study we investigated the effects of ginsenoside Rb1 (Rb1), the main active ingredients of Panax notoginseng, in alleviating podocyte injury in diabetic nephropathy and the underlying mechanisms. In cultured mouse podocyte cells, Rb1 (10 μM) significantly inhibited high glucose-induced cell apoptosis and mitochondrial injury. Furthermore, Rb1 treatment reversed high glucose-induced increases in Cyto c, Caspase 9 and mitochondrial regulatory protein NOX4, but did not affect the upregulated expression of aldose reductase (AR). Molecular docking analysis revealed that Rb1 could combine with AR and inhibited its activity. We compared the effects of Rb1 with eparestat, a known aldose reductase inhibitor, in high glucose-treated podocytes, and found that both alleviated high glucose-induced cell apoptosis and mitochondrial damage, and Rb1 was more effective in inhibiting apoptosis. In AR-overexpressing podocytes, Rb1 (10 μM) inhibited AR-mediated ROS overproduction and protected against high glucose-induced mitochondrial injury. In streptozotocin-induced DKD mice, administration of Rb1 (40 mg·kg−1·d−1, ig, for 7 weeks) significantly mitigated diabetic-induced glomerular injuries, such as glomerular hypertrophy and mesangial matrix expansion, and reduced the expression of apoptotic proteins. Collectively, Rb1 combines with AR to alleviate high glucose-induced podocyte apoptosis and mitochondrial damage, and effectively mitigates the progression of diabetic kidney disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–40.
Association AD. Microvascular complications and foot care. Diabetes Care. 2018;41:S105–18.
Association AD. 10. Microvascular complications and foot care:standards of medical care in diabetes—2018. Diabetes Care. 2017;41:S105–18.
Torban E, Braun F, Wanner N, Takano T, Goodyer PR, Lennon R, et al. From podocyte biology to novel cures for glomerular disease. Kidney Int. 2019;96:850–61.
Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82:1010–7.
Ito Y, Hsu MF, Bettaieb A, Koike S, Mello A, Calvo-Rubio M, et al. Protein tyrosine phosphatase 1B deficiency in podocytes mitigates hyperglycemia-induced renal injury. Metabolism. 2017;76:56–69.
Wei X, Wei X, Lu Z, Li L, Hu Y, Sun F, et al. Activation of TRPV1 channel antagonizes diabetic nephropathy through inhibiting endoplasmic reticulum-mitochondria contact in podocytes. Metabolism. 2020;105:154182.
Pang HH, Li MY, Wang Y, Tang MK, Ma CH, Huang JM. Effect of compatible herbs on the pharmacokinetics of effective components of Panax notoginseng in Fufang Xueshuantong Capsule. J Zhejiang Univ-Science B. 2017;18:343–52.
Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H, et al. Attenuating effect of Fufang Xueshuantong Capsule on kidney function in diabetic nephropathy model. J Nat Med. 2012;67:86–97.
Yang CY, Wang J, Zhao Y, Shen L, Jiang X, Xie ZG, et al. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J Ethnopharmacol. 2010;130:231–6.
Tu QN, Dong H, Lu FE. Effects of panax notoginoside on the nephropathy in rats with type 1 diabetes mellitus. Chin J Integr Med. 2011;17:612–5.
Du YG, Wang LP, Qian JW, Zhang KN, Chai KF. Panax notoginseng saponins protect kidney from diabetes by up-regulating silent information regulator 1 and activating antioxidant proteins in rats. Chin J Integr Med. 2015;22:910–7.
Chen ZH, Li J, Liu J, Zhao Y, Zhang P, Zhang MX, et al. Saponins isolated from the root of panax notoginseng showed significant anti-diabetic effects in KK-Ay mice. Am J Chin Med 2008;36:939–51.
Sun W, Feng LY, Zhao ZJ, Liu TH, Yang MJ. Study on antioxidant effects and inhibition of podocyte apoptosis of PNS on DN rat (in Chinese). CJTCMP. 2011;26:1062–7.
Zhou JX, Ai ZM, Sun W, Wu LL, Qin LL, Li J, et al. Mechanism study of panax notoginseng saponins on protective effect for podocyte in diabetic nephropathy mice. China J Ttrad Chin Med Pharm. 2014;29:1316–21.
Shang W, Yang Y, Jiang B, Jin H, Zhou L, Liu S, et al. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci. 2007;80:618–25.
Shang W, Yang Y, Zhou L, Jiang B, Jin H, Chen M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J Endocrinol. 2008;198:561–9.
Ma X, Xie X, Zuo C, Fan J. Effects of ginsenoside Rg1 on streptozocin-induced diabetic nephropathy in rats (in Chinese). J Biomed Eng. 2010;27:342–7.
Shang W, Yang Y, Jiang B, Jin H, Zhou L, Liu S, et al. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci. 2007;80:618–25.
Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial. Diabetes Care. 2006;29:1538–44.
Crespo I, Giménez-Dejoz J, Porté S, Cousido-Siah A, Mitschler A, Podjarny A, et al. Design, synthesis, structure-activity relationships and X-ray structural studies of novel 1-oxopyrimido[4,5-c]quinoline-2-acetic acid derivatives as selective and potent inhibitors of human aldose reductase. Eur J Med Chem. 2018;152:160–74.
Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657–84.
Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–60.
Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87.
Paul M, Hemshekhar M, Kemparaju K, Girish KS. Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic Biol Med. 2019;130:196–205.
ElGamal H, Munusamy S. Aldose reductase as a drug target for treatment of diabetic nephropathy: promises and challenges. Protein Pept Lett. 2017;24:71–7.
Cohen MP. Aldose reductase, glomerular metabolism, and diabetic nephropathy. Metabolism. 1986;35:55–9.
Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem-Biol Interact. 2011;191:330–8.
Santilli F, D’Ardes D, Davi G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul Pharmacol. 2015;74:23–37.
Yama K, Sato K, Abe N, Murao Y, Tatsunami R, Tampo Y. Epalrestat increases glutathione, thioredoxin, and heme oxygenase-1 by stimulating Nrf2 pathway in endothelial cells. Redox Biol. 2015;4:87–96.
El Gamal H, Eid AH, Munusamy S. Renoprotective effects of aldose reductase inhibitor epalrestat against high glucose-induced cellular injury. BioMed Res Int. 2017;2017:1–11.
He J, Gao HX, Yang N, Zhu XD, Sun RB, Xie Y, et al. The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol Sin. 2018;40:86–97.
Zhou P, Xie W, He S, Sun Y, Meng X, Sun G, et al. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019;8:204.
Dong C, Liu P, Wang H, Dong M, Li G, Li Y. Ginsenoside Rb1 attenuates diabetic retinopathy in streptozotocin-induced diabetic rats. Acta Cir Bras. 2019;34:e201900201.
Qin L, Wang J, Zhao R, Zhang X, Mei Y. Ginsenoside-Rb1 improved diabetic cardiomyopathy through regulating calcium signaling by alleviating protein O-GlcNAcylation. J Agric Food Chem. 2019;67:14074–85.
Nan F, Sun G, Xie W, Ye T, Sun X, Zhou P, et al. Ginsenoside Rb1 mitigates oxidative stress and apoptosis induced by methylglyoxal in SH-SY5Y cells via the PI3K/Akt pathway. Mol Cellular Probes. 2019;48:101469.
Pintusophon S, Niu W, Duan XN, Olaleye OE, Huang YH, Wang FQ, et al. Intravenous formulation of Panax notoginseng root extract: human pharmacokinetics of ginsenosides and potential for perpetrating drug interactions. Acta Pharmacol Sin. 2019;40:1351–63.
So WY, Wang Y, Ng MC, Yang X, Ma RC, Lam V, et al. Aldose reductase genotypes and cardiorenal complications: an 8-year prospective analysis of 1,074 type 2 diabetic patients. Diabetes Care. 2008;31:2148–53.
Tang X, Huang M, Jiang J, Liang X, Li X, Meng R, et al. Panax notoginseng preparations as adjuvant therapy for diabetic kidney disease: a systematic review and meta-analysis. Pharm Biol. 2020;58:138–45.
Song W, Wei L, Du Y, Wang Y, Jiang S. Protective effect of ginsenoside metabolite compound K against diabetic nephropathy by inhibiting NLRP3 inflammasome activation and NF-kappaB/p38 signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Int Immunopharmacol. 2018;63:227–38.
Iso K, Tada H, Kuboki K, Inokuchi T. Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complications. 2001;15:241–4.
Hotta N, Kawamori R, Fukuda M, Shigeta Y. Aldose Reductase Inhibitor-Diabetes Complications Trial Study G. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29:1529–33.
Acknowledgements
This work was supported by the National Key Research and Development Plan of China (2018YFC1704203), National Natural Science Foundation of China (81670671, 81870491, 82070741); Science & Technology Project of Beijing, China (D171100002817002); Fostering Fund of Chinese PLA General Hospital for National Distinguished Young Scholar Science Fund (2019-JQPY-002).
Author information
Authors and Affiliations
Contributions
XMC, QH, and JYH designed research; JYH and BXC performed research; JYH wrote the paper; BXC, SYC, RL, and GYC participated in discussions and improvements related to the manuscript. JG and XMC directed the experiment.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
He, Jy., Hong, Q., Chen, Bx. et al. Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol Sin 43, 342–353 (2022). https://doi.org/10.1038/s41401-021-00788-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00788-0
Keywords
This article is cited by
-
Ginsenoside Rb1 targets the NRF2-PPARγ-ACSL4 axis to inhibit PTECs ferroptosis
Chinese Medicine (2026)
-
Panax Notoginseng Saponins Inhibit Apoptosis and Alleviate Renal Ischemia–Reperfusion Injury Through the ROCK2/NF-κB Pathway
Molecular Biotechnology (2026)
-
Astragaloside IV Relieves Mitochondrial Oxidative Stress Damage and Dysfunction in Diabetic Mice Endothelial Progenitor Cells by Regulating the GSK-3β/Nrf2 Axis
Applied Biochemistry and Biotechnology (2025)
-
Targeting programmed cell death in diabetic kidney disease: from molecular mechanisms to pharmacotherapy
Molecular Medicine (2024)
-
ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease
Cell Communication and Signaling (2024)