Abstract
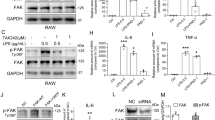
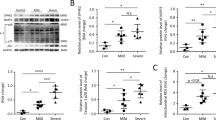
Acute lung injury (ALI) is a sudden onset systemic inflammatory response. ALI causes severe morbidity and death and currently no effective pharmacological therapies exist. Natural products represent an excellent resource for discovering new drugs. Screening anti-inflammatory compounds from the natural product bank may offer viable candidates for molecular-based therapies for ALI. In this study, 165 natural compounds were screened for anti-inflammatory activity in lipopolysaccharide (LPS)-challenged macrophages. Among the screened compounds, flavokawain B (FKB) significantly reduced LPS-induced pro-inflammatory IL-6 secretion in macrophages. FKB also reduced the formation of LPS/TLR4/MD2 complex by competitively binding to MD2, suppressing downstream MAPK and NF-κB signaling activation. Finally, FKB treatment of mice reduced LPS-induced lung injury, systemic and local inflammatory cytokine production, and macrophage infiltration in lungs. These protective activities manifested as increased survival in the ALI model, and reduced mortality upon bacterial infection. In summary, we demonstrate that the natural product FKB protects against LPS-induced lung injury and sepsis by interacting with MD2 and inhibiting inflammatory responses. FKB may potentially serve as a therapeutic option for the treatment of ALI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Probl Surg. 2020;57:100777.
Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res. 2016;167:183–91.
Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Prim. 2019;5:18.
Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4:773–83.
Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66.
Hseu YC, Chiang YC, Vudhya Gowrisankar Y, Lin KY, Huang ST, Shrestha S, et al. The in vitro and in vivo anticancer properties of chalcone flavokawain B through induction of ROS-mediated apoptotic and autophagic cell death in human melanoma cells. Cancers 2020;12:2936.
Hseu YC, Lin RW, Shen YC, Lin KY, Liao JW, Thiyagarajan V, et al. Flavokawain B and doxorubicin work synergistically to impede the propagation of gastric cancer cells via ROS-mediated apoptosis and autophagy pathways. Cancers. 2020;12:2475.
Wang J, Qi Q, Zhou W, Feng Z, Huang B, Chen A, et al. Inhibition of glioma growth by flavokawain B is mediated through endoplasmic reticulum stress induced autophagy. Autophagy. 2018;14:2007–22.
Lin CT, Senthil Kumar KJ, Tseng YH, Wang ZJ, Pan MY, Xiao JH, et al. Anti-inflammatory activity of flavokawain B from Alpinia pricei Hayata. J Agric Food Chem. 2009;57:6060–5.
Wang Y, Luo W, Han J, Khan ZA, Fang Q, Jin Y, et al. MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat Commun. 2020;11:2148.
Chen L, Chen H, Chen P, Zhang W, Wu C, Sun C, et al. Development of 2-amino-4-phenylthiazole analogues to disrupt myeloid differentiation factor 88 and prevent inflammatory responses in acute lung injury. Eur J Med Chem. 2019;161:22–38.
Su X, Wang L, Song Y, Bai C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004;30:133–40.
Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–40.
Pan H, Xu LH, Huang MY, Zha QB, Zhao GX, Hou XF, et al. Piperine metabolically regulates peritoneal resident macrophages to potentiate their functions against bacterial infection. Oncotarget. 2015;6:32468–83.
Adelusi TI, Akinbolaji GR, Yin X, Ayinde KS, Olaoba OT. Neurotrophic, anti-neuroinflammatory, and redox balance mechanisms of chalcones. Eur J Pharmacol. 2021;891:173695.
Kode J, Kovvuri J, Nagaraju B, Jadhav S, Barkume M, Sen S, et al. Synthesis, biological evaluation, and molecular docking analysis of phenstatin based indole linked chalcones as anticancer agents and tubulin polymerization inhibitors. Bioorg Chem. 2020;105:104447.
Zhang C, Yue H, Sun P, Hua L, Liang S, Ou Y, et al. Discovery of chalcone analogues as novel NLRP3 inflammasome inhibitors with potent anti-inflammation activities. Eur J Med Chem. 2021;219:113417.
Elkhalifa D, Al-Hashimi I, Al Moustafa AE, Khalil A. A comprehensive review on the antiviral activities of chalcones. J Drug Target. 2021;29:403–19.
Karimi-Sales E, Mohaddes G, Alipour MR. Chalcones as putative hepatoprotective agents: Preclinical evidence and molecular mechanisms. Pharmacol Res. 2018;129:177–87.
Rocha S, Ribeiro D, Fernandes E, Freitas M. A systematic review on anti-diabetic properties of chalcones. Curr Med Chem. 2020;27:2257–321.
Lebot V, Do TK, Legendre L. Detection of flavokavins (A, B, C) in cultivars of kava (Piper methysticum) using high performance thin layer chromatography (HPTLC). Food Chem. 2014;151:554–60.
Hseu YC, Huang YC, Thiyagarajan V, Mathew DC, Lin KY, Chen SC, et al. Anticancer activities of chalcone flavokawain B from Alpinia pricei Hayata in human lung adenocarcinoma (A549) cells via induction of reactive oxygen species-mediated apoptotic and autophagic cell death. J Cell Physiol. 2019;234:17514–26.
Chang CT, Hseu YC, Thiyagarajan V, Lin KY, Way TD, Korivi M, et al. Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch Toxicol. 2017;91:3341–64.
Peluso MR, Miranda CL, Hobbs DJ, Proteau RR, Stevens JF. Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: structure-activity relationships and in silico binding to myeloid differentiation protein-2 (MD-2). Planta Med. 2010;76:1536–43.
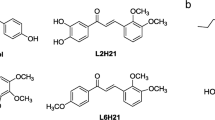
Wang Y, Shan X, Chen G, Jiang L, Wang Z, Fang Q, et al. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br J Pharmacol. 2015;172:4391–405.
Chen H, Yan T, Song Z, Ying S, Wu B, Ju X, et al. MD2 blockade prevents modified LDL-induced retinal injury in diabetes by suppressing NADPH oxidase-4 interaction with Toll-like receptor-4. Exp Mol Med. 2021;53:681–94.
Wang Y, Fang Q, Jin Y, Liu Z, Zou C, Yu W, et al. Blockade of myeloid differentiation 2 attenuates diabetic nephropathy by reducing activation of the renin-angiotensin system in mouse kidneys. Br J Pharmacol. 2019;176:2642–57.
Liu Z, Chen L, Yu P, Zhang Y, Fang B, Wu C, et al. Discovery of 3-(Indol-5-yl)-indazole derivatives as novel myeloid differentiation protein 2/toll-like receptor 4 antagonists for treatment of acute lung injury. J Med Chem. 2019;62:5453–69.
Ouyang W, Zhou H, Liu C, Wang S, Han Y, Xia J, et al. 25-Hydroxycholesterol protects against acute lung injury via targeting MD-2. J Cell Mol Med. 2018;22:5494–503.
Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–72.
Hadina S, Weiss JP, McCray PB Jr, Kulhankova K, Thorne PS. MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharides: effect of acylation state. Am J Respir Cell Mol Biol. 2008;38:647–54.
Pugin J, Stern-Voeffray S, Daubeuf B, Matthay MA, Elson G, Dunn-Siegrist I. Soluble MD-2 activity in plasma from patients with severe sepsis and septic shock. Blood. 2004;104:4071–9.
Yang Y, Han C, Sheng Y, Wang J, Zhou X, Li W, et al. The mechanism of aureusidin in suppressing inflammatory response in acute liver injury by regulating MD2. Front Pharmacol. 2020;11:570776.
Wu P, Yan H, Qi J, Jia W, Zhang W, Yao D, et al. L6H9 attenuates LPS-induced acute lung injury in rats through targeting MD2. Drug Dev Res. 2020;81:85–92.
Chen H, Zhang Y, Zhang W, Liu H, Sun C, Zhang B, et al. Inhibition of myeloid differentiation factor 2 by baicalein protects against acute lung injury. Phytomedicine. 2019;63:152997.
Zhang Y, Xu T, Pan Z, Ge X, Sun C, Lu C, et al. Shikonin inhibits myeloid differentiation protein 2 to prevent LPS-induced acute lung injury. Br J Pharmacol. 2018;175:840–54.
Hosoki K, Boldogh I, Aguilera-Aguirre L, Sun Q, Itazawa T, Hazra T, et al. Myeloid differentiation protein 2 facilitates pollen- and cat dander-induced innate and allergic airway inflammation. J Allergy Clin Immunol. 2016;137:1506–13.
Cheng P, Li S, Chen H. Macrophages in lung injury, repair, and fibrosis. Cells. 2021;10:436.
Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (81970338 to YW, 82070833 to CZ, and 82000793 to WL), Natural Science Foundation of Zhejiang Province (LR18H160003 to YW), and Zhejiang Provincial Key Research and Development Program (2021C03070 to CZ).
Author information
Authors and Affiliations
Contributions
WL, LBY, CCQ, BM, GMM, XMM, JW, CHH, BJ, and LXZ: collection, analysis, and interpretation of data; YW, CZ, and GL: conception and design, interpretation of data, manuscript writing, and manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Luo, W., Yang, Lb., Qian, Cc. et al. Flavokawain B alleviates LPS-induced acute lung injury via targeting myeloid differentiation factor 2. Acta Pharmacol Sin 43, 1758–1768 (2022). https://doi.org/10.1038/s41401-021-00792-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00792-4
Keywords
This article is cited by
-
Bisdemethoxycurcumin alleviates LPS-induced acute lung injury via activating AMPKα pathway
BMC Pharmacology and Toxicology (2023)