Abstract
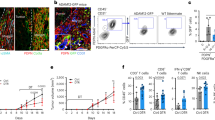
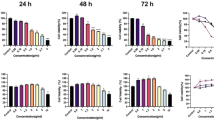
Hydrogen sulfide (H2S) is widely recognized as the third endogenous gas signaling molecule and may play a key role in cancer biological processes. ADT-OH (5-(4-hydroxyphenyl)−3H-1,2-dithiocyclopentene-3-thione) is one of the most widely used organic donors for the slow release of H2S and considered to be a potential anticancer compound. In this study, we investigated the antimetastatic effects of ADT-OH in highly metastatic melanoma cells. A tail-vein-metastasis model was established by injecting B16F10 and A375 cells into the tail veins of mice, whereas a mouse footpad-injection model was established by injecting B16F10 cells into mouse footpads. We showed that administration of ADT-OH significantly inhibited the migration and invasion of melanoma cells in the three different animal models. We further showed that ADT-OH dose-dependently inhibited the migration and invasion of B16F10, B16F1 and A375 melanoma cells as evaluated by wound healing and Transwell assays in vitro. LC-MS/MS and bioinformatics analyses revealed that ADT-OH treatment inhibited the EMT process in B16F10 and A375 cells by reducing the expression of FAK and the downstream response protein Paxillin. Overexpression of FAK reversed the inhibitory effects of ADT-OH on melanoma cell migration. Moreover, after ADT-OH treatment, melanoma cells showed abnormal expression of the H2S-producing enzymes CSE/CBS and the AKT signaling pathways. In addition, ADT-OH significantly suppressed the proliferation of melanoma cells. Collectively, these results demonstrate that ADT-OH inhibits the EMT process in melanoma cells by suppressing the CSE/CBS and FAK signaling pathways, thereby exerting its antimetastatic activity. ADT-OH may be used as an antimetastatic agent in the future.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Geller AC, Clapp RW, Sober AJ, Gonsalves L, Mueller L, Christiansen CL, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172–8.
Palrasu M, Knapinska AM, Diez J, Smith L, LaVoi T, Giulianotti M, et al. A novel probe for spliceosomal proteins that induces autophagy and death of melanoma cells reveals new targets for melanoma drug discovery. Cell Physiol Biochem. 2019;53:656–86.
Szabo C, Coletta C, Chao C, Modis K, Szczesny B, Papapetropoulos A, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA. 2013;110:12474–9.
Arribillaga L, Dotor J, Basagoiti M, Riezu-Boj JI, Borras-Cuesta F, Lasarte JJ, et al. Therapeutic effect of a peptide inhibitor of TGF-beta on pulmonary fibrosis. Cytokine. 2011;53:327–33.
Lu S, Gao Y, Huang X, Wang X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int J Oncol. 2014;44:1259–67.
Murata T, Sato T, Kamoda T, Moriyama H, Kumazawa Y, Hanada N. Differential susceptibility to hydrogen sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer cell lines and oral keratinocytes: role of PHLDA1 as an apoptosis suppressor. Exp Cell Res. 2014;320:247–57.
Panza E, De Cicco P, Armogida C, Scognamiglio G, Gigantino V, Botti G, et al. Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28:61–72.
Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85:689–703.
Ngowi EE, Afzal A, Sarfraz M, Khattak S, Zaman SU, Khan NH, et al. Role of hydrogen sulfide donors in cancer development and progression. Int J Biol Sci. 2021;17:73–88.
Wu D, Si W, Wang M, Lv S, Ji A, Li Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 2015;50:38–45.
Fan K, Li N, Qi J, Yin P, Zhao C, Wang L, et al. Wnt/beta-catenin signaling induces the transcription of cystathionine-gamma-lyase, a stimulator of tumor in colon cancer. Cell Signal. 2014;26:2801–8.
Olah G, Modis K, Toro G, Hellmich MR, Szczesny B, Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem Pharmacol. 2018;149:186–204.
Wang RH, Chu YH, Lin KT. The hidden role of hydrogen sulfide metabolism in cancer. Int J Mol Sci. 2021;22:6562.
Giuffre A, Tome CS, Fernandes DGF, Zuhra K, Vicente JB. Hydrogen sulfide metabolism and signaling in the tumor microenvironment. Adv Exp Med Biol. 2020;1219:335–53.
De Cicco P, Panza E, Ercolano G, Armogida C, Sessa G, Pirozzi G, et al. ATB-346, a novel hydrogen sulfide-releasing anti-inflammatory drug, induces apoptosis of human melanoma cells and inhibits melanoma development in vivo. Pharmacol Res. 2016;114:67–73.
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:e21077.
Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem Toxicol. 2013;57:362–70.
Lee ZW, Teo XY, Tay EY, Tan CH, Hagen T, Moore PK, et al. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br J Pharmacol. 2014;171:4322–36.
Lv M, Li Y, Ji MH, Zhuang M, Tang JH. Inhibition of invasion and epithelial-mesenchymal transition of human breast cancer cells by hydrogen sulfide through decreased phospho-p38 expression. Mol Med Rep. 2014;10:341–6.
Jiang W, Cai F, Xu H, Lu Y, Chen J, Liu J, et al. Extracellular signal regulated kinase 5 promotes cell migration, invasion and lung metastasis in a FAK-dependent manner. Protein Cell. 2020;11:825–45.
Cai F, Chen M, Zha D, Zhang P, Zhang X, Cao N, et al. Curcumol potentiates celecoxib-induced growth inhibition and apoptosis in human non-small cell lung cancer. Oncotarget. 2017;8:115526–45.
Jiang W, Jin G, Cai F, Chen X, Cao N, Zhang X, et al. Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Exp Mol Med. 2019;51:1–20.
Cai F, Xu H, Cao N, Zhang X, Liu J, Lu Y, et al. ADT-OH, a hydrogen sulfide-releasing donor, induces apoptosis and inhibits the development of melanoma in vivo by upregulating FADD. Cell Death Dis. 2020;11:33.
Guo H, Gai JW, Wang Y, Jin HF, Du JB, Jin J. Characterization of hydrogen sulfide and its synthases, cystathionine beta-synthase and cystathionine gamma-lyase, in human prostatic tissue and cells. Urology. 2012;79:483 e1–5.
Zhang L, Qi Q, Yang J, Sun D, Li C, Xue Y, et al. An anticancer role of hydrogen sulfide in human gastric cancer cells. Oxid Med Cell Longev. 2015;2015:636410.
Jurkowska H, Placha W, Nagahara N, Wrobel M. The expression and activity of cystathionine-gamma-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids. 2011;41:151–8.
Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Cheng Y, Gao XH, Li XJ, Cao QH, Zhao DD, Zhou JR, et al. Depression promotes prostate cancer invasion and metastasis via a sympathetic-cAMP-FAK signaling pathway. Oncogene. 2018;37:2953–66.
Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18:18.
Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35:S244–S75. Suppl
Mishra H, Mishra PK, Ekielski A, Jaggi M, Iqbal Z, Talegaonkar S. Melanoma treatment: from conventional to nanotechnology. J Cancer Res Clin Oncol. 2018;144:2283–302.
Pal M. Tumor metastasis suppressor functions of Ets transcription factor through integrin beta3-mediated signaling pathway. J Cell Physiol. 2019;234:20266–74.
Chen S, Yue T, Huang Z, Zhu J, Bu D, Wang X, et al. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5-FU through miR-215-5p-EREG/TYMS axis in colon cancer cells. Cancer Lett. 2019;466:49–60.
Sakuma S, Minamino S, Takase M, Ishiyama Y, Hosokura H, Kohda T, et al. Hydrogen sulfide donor GYY4137 suppresses proliferation of human colorectal cancer Caco-2 cells by inducing both cell cycle arrest and cell death. Heliyon. 2019;5:e02244.
Yang CT, Chen L, Xu S, Day JJ, Li X, Xian M. Recent development of hydrogen sulfide releasing/stimulating reagents and their potential applications in cancer and glycometabolic disorders. Front Pharmacol. 2017;8:664.
Chattopadhyay M, Kodela R, Nath N, Barsegian A, Boring D, Kashfi K. Hydrogen sulfide-releasing aspirin suppresses NF-kappaB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2012;83:723–32.
Bai YW, Ye MJ, Yang DL, Yu MP, Zhou CF, Shen T. Hydrogen sulfide attenuates paraquat-induced epithelial-mesenchymal transition of human alveolar epithelial cells through regulating transforming growth factor-beta1/Smad2/3 signaling pathway. J Appl Toxicol. 2019;39:432–40.
Li D, Yang Z, Liu Z, Zou Q, Yuan Y. Clinical significance of CBS and CCL21 in gallbladder adenocarcinomas and squamous cell/adenosquamous carcinomas. Appl Immunohistochem Mol Morphol. 2020;28:103–10.
Wang YH, Huang JT, Chen WL, Wang RH, Kao MC, Pan YR, et al. Dysregulation of cystathionine gamma-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20:e45986.
Hasegawa U, Tateishi N, Uyama H, van der Vlies AJ. Hydrolysis-sensitive dithiolethione prodrug micelles. Macromol Biosci. 2015;15:1512–22.
Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216:1016–26.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–26.
Tulchinsky E, Demidov O, Kriajevska M, Barlev NA, Imyanitov E. EMT: a mechanism for escape from EGFR-targeted therapy in lung cancer. Biochim Biophys Acta Rev Cancer. 2019;1871:29–39.
He X, Xue M, Jiang S, Li W, Yu J, Xiang S. Fucoidan promotes apoptosis and inhibits EMT of breast cancer cells. Biol Pharm Bull. 2019;42:442–7.
Cho ES, Kang HE, Kim NH, Yook JI. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharmacol Res. 2019;42:14–24.
Liao CC, Chen YH, Lin F, Qi YF. Hydrogen sulfide inhibits transforming growth factor beta-1 induced bronchial epithelial-mesenchymal transition. Chin Med J (Engl). 2015;128:3247–50.
Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89:140–5.
Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67.
Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA. 2007;104:20302–7.
Yu HG, Nam JO, Miller NL, Tanjoni I, Walsh C, Shi L, et al. p190RhoGEF (Rgnef) promotes colon carcinoma tumor progression via interaction with focal adhesion kinase. Cancer Res. 2011;71:360–70.
Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell. 2011;22:327–41.
Fang LP, Lin Q, Tang CS, Liu XM. Hydrogen sulfide attenuates epithelial-mesenchymal transition of human alveolar epithelial cells. Pharmacol Res. 2010;61:298–305.
Cheng S, Lu Y, Li Y, Gao L, Shen H, Song K. Hydrogen sulfide inhibits epithelial-mesenchymal transition in peritoneal mesothelial cells. Sci Rep. 2018;8:5863.
Acknowledgements
This study was supported by grants from the Chinese National Natural Sciences Foundation (81773099, 81630092 and 82130106), Department of Science and Technology of Jiangsu Province (BK20192005) and the program B for Outstanding PhD candidate of Nanjing University (202001B051).
Author information
Authors and Affiliations
Contributions
ZCH, HQZ and JC (Jian Cheng) designed the outline of the paper. HQZ revised this paper. FFC and HRX contributed equally to this work. FFC and HRX performed most of the experiments in this study. FFC wrote the paper and prepared the Figures. SHY, PL, YYL, JC (Jia Chen), ZQB and HSS helped with the cell related experiments. SHY and PL helped with the experiments using animals. All authors have read and approved the final version of this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Animal welfare and experimental procedures were performed in strict accordance with high standard animal welfare and other related ethical regulations approved by the Nanjing University Animal Care and Use Committee.
Supplementary information
Rights and permissions
About this article
Cite this article
Cai, Ff., Xu, Hr., Yu, Sh. et al. ADT-OH inhibits malignant melanoma metastasis in mice via suppressing CSE/CBS and FAK/Paxillin signaling pathway. Acta Pharmacol Sin 43, 1829–1842 (2022). https://doi.org/10.1038/s41401-021-00799-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00799-x
Keywords
This article is cited by
-
Sulfide regulation and catabolism in health and disease
Signal Transduction and Targeted Therapy (2025)
-
ADT-OH exhibits anti-metastatic activity on triple-negative breast cancer by combinatorial targeting of autophagy and mitochondrial fission
Cell Death & Disease (2024)