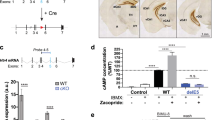
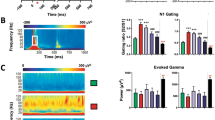
Abstract
Hypidone hydrochloride (YL-0919) is a novel antidepressant in clinical phase II trial. Previous studies show that YL-0919 is a selective 5-HT (serotonin) reuptake inhibitor, 5-HT1A receptor partial agonist, and 5-HT6 receptor agonist, which exerts antidepressant effects in various animal models, but its effects on neural function remain unclear. Medial prefrontal cortex (mPFC), a highly evolved brain region, controls highest order cognitive functions and emotion regulation. In this study we investigated the effects of YL-0919 on the mPFC function, including the changes in neuronal activities using electrophysiological recordings. Extracellular recording (in vivo) showed that chronic administration of YL-0919 significantly increased the spontaneous discharges of mPFC neurons. In mouse mPFC slices, whole-cell recording revealed that perfusion of YL-0919 significantly increased the frequency of sEPSCs, but decreased the frequency of sIPSCs. Then we conducted whole-cell recording in mPFC slices of GAD67-GFP transgenic mice, and demonstrated that YL-0919 significantly inhibited the excitability of GABAergic neurons. In contrast, it did not alter the excitability of pyramidal neurons in mPFC slices of normal mice. Moreover, the inhibition of GABAergic neurons by YL-0919 was prevented by pre-treatment with 5-HT1A receptor antagonist WAY 100635. Finally, chronic administration of YL-0919 significantly increased the phosphorylation levels of mTOR and GSK-3β in the mPFC as compared with vehicle. Taken together, our results demonstrate that YL-0919 enhances the excitability of mPFC via a disinhibition mechanism to fulfill its rapid antidepressant neural mechanism, which was accomplished by 5-HT1A receptor-mediated inhibition of inhibitory GABAergic interneurons.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH, Yan S, et al. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin. 2016;37:1141–53.
Chu SF, Zhang Z, Zhou X, He WB, Yang B, Cui LY, et al. Low corticosterone levels attenuate late life depression and enhance glutamatergic neurotransmission in female rats. Acta Pharmacol Sin. 2021;42:848–60.
Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19.
Yin YY, Tian CY, Fang XX, Shang C, Zhang LM, Xu Q, et al. The faster-onset antidepressant effects of hypidone hydrochloride (YL-0919) in monkeys subjected to chronic unpredictable stress. Front Pharmacol. 2020;11:5868–79.
Pan SJ, Tan YL, Yao SW, Xin Y, Yang X, Liu J, et al. Fluoxetine induces lipid metabolism abnormalities by acting on the liver in patients and mice with depression. Acta Pharmacol Sin. 2018;39:1463–72.
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82:549–59.
Li YF. A hypothesis of monoamine (5-HT) – glutamate/GABA long neural circuit: aiming for fast-onset antidepressant discovery. Pharmacol Ther. 2020;208:107494.
McCormack PL. Vilazodone: a review in major depressive disorder in adults. Drugs. 2015;75:1915–23.
Wagner G, Schultes MT, Titscher V, Teufer B, Klerings I, Gartlehner G. Efficacy and safety of levomilnacipran, vilazodone and vortioxetine compared with other second-generation antidepressants for major depressive disorder in adults:a systematic review and network meta-analysis. J Affect Disord. 2018;228:1–12.
Dixon ML, Thiruchselvam R, Todd R, Christoff K. Emotion and the prefrontal cortex: an integrative review. Psychological Bull. 2017;143:1033–81.
Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–32.
Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107.
Lee YA, Poirier P, Otani S, Goto Y. Dorsal-ventral distinction of chronic stress-induced electrophysiological alterations in the rat medial prefrontal cortex. Neuroscience. 2011;183:108–20.
Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25:530–43.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109.
McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. “Braking” the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry. 2019;86:669–81.
Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–9.
Ran YH, Hu XX, Wang YL, Zhao N, Zhang LM, Liu HX, et al. YL-0919, a dual 5-HT1A partial agonist and SSRI, produces antidepressant- and anxiolytic-like effects in rats subjected to chronic unpredictable stress. Acta Pharmacol Sin. 2018;39:12–23.
Chen XF, Jin ZL, Gong Y, Zhao N, Wang XY, Ran YH, et al. 5-HT6 receptor agonist and memory-enhancing properties of hypidone hydrochloride (YL-0919), a novel 5-HT1Areceptor partial agonist and SSRI. Neuropharmacology. 2018;138:1–9.
Sun LJ, Zhang LM, Liu D, Xue R, Liu YQ, Li L, et al. The faster-onset antidepressant effects of hypidone hydrochloride (YL-0919). Metab Brain Dis. 2019;34:1375–84.
Zhang LM, Wang XY, Zhao N, Wang YL, Hu XX, Ran YH, et al. Neurochemical and behavioural effects of hypidone hydrochloride (YL-0919): a novel combined selective 5-HT reuptake inhibitor and partial 5-HT(1A) agonist. Br J Pharmacol. 2017;174:769–80.
Ran Y, Jin Z, Chen X, Zhao N, Fang X, Zhang L, et al. Hypidone hydrochloride (YL-0919) produces a fast-onset reversal of the behavioral and synaptic deficits caused by chronic stress exposure. Front Cell Neurosci. 2018;12:395.
Suman PR, Zerbinatti N, Theindl LC, Domingues K, Lino de Oliveira C. Failure to detect the action of antidepressants in the forced swim test in Swiss mice. Acta Neuropsychiatr. 2018;30:158–67.
Li Q, Zhang B, Cao H, Liu W, Guo F, Shen F, et al. Oxytocin exerts antidepressant-like effect by potentiating dopaminergic synaptic transmission in the mPFC. Neuropharmacology. 2020;162:107836.
Guo F, Zhang Q, Zhang B, Fu Z, Wu B, Huang C, et al. Burst-firing patterns in the prefrontal cortex underlying the neuronal mechanisms of depression probed by antidepressants. Eur J Neurosci. 2014;40:3538–47.
Legéndy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–39.
Helboe L, Egebjerg J, de Jong IE. Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: a double in situ hybridization study. Neuroscience. 2015;310:442–54.
Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–99.
Zhou X, Zhang R, Zhang S, Wu J, Sun X. Activation of 5-HT1A receptors promotes retinal ganglion cell function by inhibiting the cAMP-PKA pathway to modulate presynaptic GABA release in chronic glaucoma. J Neurosci. 2019;39:1484–504.
Normann C, Frase S, Haug V, von Wolff G, Clark K, Münzer P, et al. Antidepressants rescue stress-induced disruption of synaptic plasticity via serotonin transporter-independent inhibition of L-type calcium channels. Biol Psychiatry. 2018;84:55–64.
Cavaccini A, Gritti M, Giorgi A, Locarno A, Heck N, Migliarini S, et al. Serotonergic signaling controls input-specific synaptic plasticity at striatal circuits. Neuron. 2018;98:801–16.e7.
Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6.
Lei Y, Wang J, Wang D, Li C, Liu B, Fang X, et al. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex. Mol Psychiatry. 2020;25:1094–111.
Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 2019;10:223.
Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2017;22:920–30.
Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci USA. 2018;115:E3007–e16.
Fogaça MV, Wu M, Li C, Li XY, Picciotto MR, Duman RS. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol Psychiatry. 2021;26:3277–91.
Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203.
Borroto-Escuela DO, Narváez M, Ambrogini P, Ferraro L, Brito I, Romero-Fernandez W, et al. Receptor-receptor interactions in multiple 5-HT1A heteroreceptor complexes in raphe-hippocampal 5-HT transmission and their televance for depression and its treatment. Molecules. 2018;23:1341.
Lladó-Pelfort L, Santana N, Ghisi V, Artigas F, Celada P. 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex. 2012;22:1487–97.
Gorinski N, Bijata M, Prasad S, Wirth A, Abdel Galil D, Zeug A, et al. Attenuated palmitoylation of serotonin receptor 5-HT1A affects receptor function and contributes to depression-like behaviors. Nat Commun. 2019;10:3924.
Linge R, Jiménez-Sánchez L, Campa L, Pilar-Cuéllar F, Vidal R, Pazos A, et al. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26.
Fukumoto K, Iijima M, Funakoshi T, Chaki S. Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol. 2018;21:371–81.
Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774–8.
Renner U, Zeug A, Woehler A, Niebert M, Dityatev A, Dityateva G, et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J Cell Sci. 2012;125:2486–99.
Łukasiewicz S, Błasiak E, Szafran-Pilch K, Dziedzicka-Wasylewska M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics - in vitro studies. J Neurochem. 2016;137:549–60.
Borroto-Escuela DO, Tarakanov AO, Fuxe K. FGFR1-5-HT1A heteroreceptor complexes: implications for understanding and treating major depression. Trends Neurosci. 2016;39:5–15.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Wang H, Huang B, Wang W, Li J, Chen Y, Flynn T, et al. High urea induces depression and LTP impairment through mTOR signalling suppression caused by carbamylation. EBioMedicine. 2019;48:478–90.
Ma K, Yang LM, Chen HZ, Lu Y. Activation of muscarinic receptors inhibits glutamate-induced GSK-3β overactivation in PC12 cells. Acta Pharmacol Sin. 2013;34:886–92.
Xu ZX, Tan JW, Xu H, Hill CJ, Ostrovskaya O, Martemyanov KA, et al. Caspase-2 promotes AMPA receptor internalization and cognitive flexibility via mTORC2-AKT-GSK3β signaling. Nat Commun. 2019;10:3622.
Acknowledgements
We thank Dr Shu-jia Zhu (Institute of Neuroscience, China) who provided the GAD-GFP mice. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12040220), the National Natural Science Foundation of China (31671049, 81773708, 81072624, and 81173036), and the National Key New Drug Creation Program of China (No. 2017ZX09309012, 2018ZX09739008, and 2018ZX09711002-002-012).
Author information
Authors and Affiliations
Contributions
YL and YFL directed and supervised the project. YMZ performed experiments and analyzed data. LYY helped to produce transgenic mice and the extracellular electrophysiological recording. TYL contributed to the R-scope procedures. Fan Guo assisted with behavioral experiments. YMZ, YFL, YL, and Fei Guo designed the study and wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Ym., Ye, Ly., Li, Ty. et al. New monoamine antidepressant, hypidone hydrochloride (YL-0919), enhances the excitability of medial prefrontal cortex in mice via a neural disinhibition mechanism. Acta Pharmacol Sin 43, 1699–1709 (2022). https://doi.org/10.1038/s41401-021-00807-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00807-0
Keywords
This article is cited by
-
Androgens alleviate the depression-like phenotype in female mice by inhibiting AVPR1a in the hippocampal brain region
Molecular Medicine (2025)
-
The DRN-VLO pathway via distinct 5-HT synaptic mechanisms modulates neuropathic pain–induced depressive-like behaviors in mice
The Journal of Headache and Pain (2025)
-
Sex-specific and developmental effects of early life adversity on stress reactivity are rescued by postnatal knockdown of 5-HT1A autoreceptors
Neuropsychopharmacology (2025)
-
Modulation of neuroimmune cytokine networks by antidepressants: implications in mood regulation
Translational Psychiatry (2025)
-
The development and benefits of metformin in various diseases
Frontiers of Medicine (2023)