Abstract
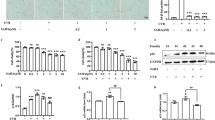
Excessive exposure to UVB induces skin diseases. Silibinin, a flavonolignan used for treating liver diseases, is found to be effective against UVB-caused skin epidermal and dermal cell damage. In this study we investigated the molecular mechanisms underlying. Human nonmalignant immortalized keratinocyte HaCaT cells and neonatal human foreskin fibroblasts HFFs were exposed to UVB irradiation. We showed that pre-treatment with silibinin dose-dependently decreased UVB-induced apoptosis of HaCaT cells. Furthermore, we showed that silibinin treatment inhibited nuclear translocation of YAP after UVB irradiation. Molecular docking analysis and DARTS assay confirmed the direct interaction of silibinin with YAP. Silencing YAP by siRNA had no influence on the survival of HaCaT cells, whereas inhibiting classical YAP-TEAD signaling pathway by siRNA targeting TEAD1 or its pharmaceutical inhibitor verteporfin further augmented UVB-induced apoptosis, suggesting that YAP-TEAD pathway was prosurvival, which did not participate in the protective effect of silibinin. We then explored the pro-apoptotic YAP-p73 pathway. p73 was upregulated in UVB-irradiated cells, but reduced by silibinin cotreatment. The mRNA and protein levels of p73 target genes (PML, p21 and Bax) were all increased by UVB but decreased by silibinin co-treatment. Inhibiting p73 by using siRNA reduced UVB-induced apoptosis, suggesting that downregulation of p73 was responsible for the cytoprotective effect of silibinin. In HFFs, the upregulated YAP-p73 pathway by UVB irradiation was also suppressed by silibinin. Collectively, YAP-p73 pathway is a major cause of the death of UVB-exposed epidermal HaCaT cells and dermal HFFs. Silibinin directly inhibits YAP-p73 pathway, exerting the protective action on UVB-irradiated skin cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hart PH, Norval M, Byrne SN, Rhodes LE. Exposure to ultraviolet radiation in the modulation of human diseases. Annu Rev Pathol. 2019;14:55–81.
Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466–79.
Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol. 2019;19:688–701.
Baek JY, Park S, Park J, Jang JY, Wang SB, Kim SR, et al. Protective role of mitochondrial peroxiredoxin III against UVB-induced apoptosis of epidermal keratinocytes. J Invest Dermatol. 2017;137:1333–42.
Sudol M, Bork P, Einbond A, Kastury K, Druck T, Negrini M, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–41.
Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–52.
Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20:888–99.
Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–312.
Furth N, Aylon Y, Oren M. p53 shades of Hippo. Cell Death Differ. 2018;25:81–92.
Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–73.
Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell. 2005;18:447–59.
Perez Gonzalez N, Tao J, Rochman ND, Vig D, Chiu E, Wirtz D, et al. Cell tension and mechanical regulation of cell volume. Mol Biol Cell. 2018;29:0.
Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–70.
Biedermann D, Vavrikova E, Cvak L, Kren V. Chemistry of silybin. Nat Prod Rep. 2014;31:1138–57.
Si L, Liu W, Hayashi T, Ji Y, Fu J, Nie Y, et al. Silibinin-induced apoptosis of breast cancer cells involves mitochondrial impairment. Arch Biochem Biophys. 2019;671:42–51.
Liu B, Liu W, Liu P, Liu X, Song X, Hayashi T, et al. Silibinin alleviates the learning and memory defects in overtrained rats accompanying reduced neuronal apoptosis and senescence. Neurochem Res. 2019;44:1818–29.
Liu W, Otkur W, Zhang Y, Li Q, Ye Y, Zang L, et al. Silibinin protects murine fibroblast L929 cells from UVB-induced apoptosis through the simultaneous inhibition of ATM-p53 pathway and autophagy. FEBS J. 2013;280:4572–84.
Wang Q, Ye Y, Liu W, Jiang S, Tashiro S-I, Onodera S, et al. Dual effects of silibinin treatment on autophagy-regulated dermal apoptosis retardation and epidermal apoptosis up-regulation in UVB-induced skin inflammation. J Asian Nat Prod Res. 2012;14:688–99.
Rigby CM, Roy S, Deep G, Guillermo-Lagae R, Jain AK, Dhar D, et al. Role of p53 in silibinin-mediated inhibition of ultraviolet B radiation-induced DNA damage, inflammation and skin carcinogenesis. Carcinogenesis. 2017;38:40–50.
Liu W, Wang F, Li C, Otkur W, Hayashi T, Mizuno K, et al. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J Photoch Photobio B. 2021;216:112147.
Hussain RN, Jmor F, Damato B, Heimann H. Verteporfin photodynamic therapy for the treatment of retinal vasoproliferative tumors. Ophthalmology. 2015;122:2361–3.
Ratkay LG, Waterfield JD, Hunt DW. Photodynamic therapy in immune (non-oncological) disorders: focus on benzoporphyrin derivatives. BioDrugs. 2000;14:127–35.
Otkur W, Wang F, Liu W, Hayashi T, Tashiro SI, Onodera S, et al. Persistent IKKalpha phosphorylation induced apoptosis in UVB and Poly I:C co-treated HaCaT cells plausibly through pro-apoptotic p73 and abrogation of IkappaBalpha. Mol Immunol. 2018;104:69–78.
Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, et al. Target identification using drug affinity responsive target stability (DARTS). P Natl Acad Sci USA. 2009;106:21984–9.
Chin RM, Fu XD, Pai MY, Vergnes L, Hwang H, Deng G, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401.
Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–82.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513.
Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–91.
Kaneko KJ, DePamphilis ML. Regulation of gene expression at the beginning of mammalian development and the TEAD family of transcription factors. Dev Genet. 1998;22:43–55.
Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G, et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803–14.
Downward J, Basu S. YAP and p73: a complex affair. Mol Cell. 2008;32:749–50.
Lin KC, Park HW, Guan KL. Regulation of the Hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42:862–72.
Papaspyropoulos A, Bradley L, Thapa A, Leung CY, Toskas K, Koennig D, et al. RASSF1A uncouples Wnt from Hippo signalling and promotes YAP mediated differentiation via p73. Nat Commun. 2018;9:424.
Tomlinson V, Gudmundsdottir K, Luong P, Leung KY, Knebel A, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29.
Lee KK, Yonehara S. Identification of mechanism that couples multisite phosphorylation of Yes-associated protein (YAP) with transcriptional coactivation and regulation of apoptosis. J Biol Chem. 2012;287:9568–78.
Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–410 e14.
Bai ZL, Tay V, Guo SZ, Ren J, Shu MG. Silibinin induced human glioblastoma cell apoptosis concomitant with autophagy through simultaneous inhibition of mTOR and YAP. BioMed Res Int. 2018;2018:6165192.
Song X, Zhou B, Cui L, Lei D, Zhang P, Yao G, et al. Silibinin ameliorates Abeta25-35-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress. Neurochem Res. 2017;42:1073–83.
Song X, Zhou B, Zhang P, Lei D, Wang Y, Yao G, et al. Protective effect of silibinin on learning and memory impairment in LPS-treated rats via ROS-BDNF-TrkB pathway. Neurochem Res. 2016;41:1662–72.
Yang J, Sun Y, Xu F, Liu W, Hayashi T, Onodera S, et al. Involvement of estrogen receptors in silibinin protection of pancreatic beta-cells from TNFalpha- or IL-1beta-induced cytotoxicity. Biomed Pharmacother. 2018;102:344–53.
Yang J, Sun Y, Xu F, Liu W, Mai Y, Hayashi T, et al. Silibinin ameliorates amylin-induced pancreatic beta-cell apoptosis partly via upregulation of GLP-1R/PKA pathway. Mol Cell Biochem. 2019;452:83–94.
Li LH, Wu LJ, Jiang YY, Tashiro S, Onodera S, Uchiumi F, et al. Silymarin enhanced cytotoxic effect of anti-Fas agonistic antibody CH11 on A375-S2 cells. J Asian Nat Prod Res. 2007;9:593–602.
Duan WJ, Li QS, Xia MY, Tashiro S, Onodera S, Ikejima T. Silibinin activated p53 and induced autophagic death in human fibrosarcoma HT1080 cells via reactive oxygen species-p38 and c-Jun N-terminal kinase pathways. Biol Pharm Bull. 2011;34:47–53.
Fan S, Yu Y, Qi M, Sun Z, Li L, Yao G, et al. P53-mediated GSH depletion enhanced the cytotoxicity of NO in silibinin-treated human cervical carcinoma HeLa cells. Free Radic Res. 2012;46:1082–92.
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71.
Pollet M, Shaik S, Mescher M, Frauenstein K, Tigges J, Braun SA, et al. The AHR represses nucleotide excision repair and apoptosis and contributes to UV-induced skin carcinogenesis. Cell Death Differ. 2018;25:1823–36.
Skobowiat C, Brozyna AA, Janjetovic Z, Jeayeng S, Oak ASW, Kim TK, et al. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J Pineal Res. 2018;65:e12501.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81703528).
Author information
Authors and Affiliations
Contributions
WWL and TI designed the research; WWL, FW, CL, WO and YYZ performed the research; WWL and FW analyzed the data; WWL wrote the paper; XYS performed the molecular docking study; SH and HF contributed to cell culture and contributed some reagents; and TI, TH and KM revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Liu, Ww., Wang, F., Li, C. et al. Silibinin relieves UVB-induced apoptosis of human skin cells by inhibiting the YAP-p73 pathway. Acta Pharmacol Sin 43, 2156–2167 (2022). https://doi.org/10.1038/s41401-021-00826-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00826-x
Keywords
This article is cited by
-
On the mechanisms of epidermal stemness and differentiation
npj Systems Biology and Applications (2025)
-
Molecular approaches to prevent UV-induced premature skin aging: focus on phytochemicals as photo-protectants
Phytochemistry Reviews (2025)
-
Immunoprotective effect of silybin through blocking p53-driven caspase-9-Apaf-1-Cyt c complex formation and immune dysfunction after difenoconazole exposure in carp spleen
Environmental Science and Pollution Research (2024)
-
Specific Knockdown of the NDUFS4 Gene Reveals Important Roles of Ferroptosis in UVB-induced Photoaging
Inflammation (2024)
-
The biology of YAP in programmed cell death
Biomarker Research (2022)