Abstract
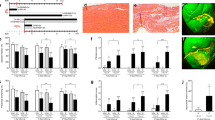
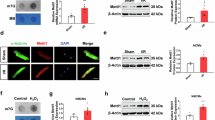
We previously found that the levels of metabolite N-acetylglutamine were significantly increased in urine samples of patients with heart failure (HF) and in coronary artery ligation (CAL)-induced HF mice, whereas the expression of its specific metabolic-degrading enzyme aminoacylase-1 (ACY1) was markedly decreased. In the current study, we investigated the role of ACY1 in the pathogenesis of HF and the therapeutic effects of 20(S)-ginsenoside Rg3 in HF experimental models in vivo and in vitro. HF was induced in mice by CAL. The mice were administered Rg3 (7.5, 15, 30 mg · kg−1· d−1, i.g.), or positive drug metoprolol (Met, 5.14 mg · kg−1· d−1, i.g.), or ACY1 inhibitor mono-tert-butyl malonate (MTBM, 5 mg · kg−1 · d−1, i.p.) for 14 days. We showed that administration of MTBM significantly exacerbated CAL-induced myocardial injury, aggravated cardiac dysfunction, and pathological damages, and promoted myocardial fibrosis in CAL mice. In Ang II-induced mouse cardiac fibroblasts (MCFs) model, overexpression of ACY1 suppressed the expression of COL3A1 and COL1A via inhibiting TGF-β1/Smad3 pathway, whereas ACY1-siRNA promoted the cardiac fibrosis responses. We showed that a high dose of Rg3 (30 mg · kg−1· d−1) significantly decreased the content of N-acetylglutamine, increased the expression of ACY1, and inhibited TGF-β1/Smad3 pathway in CAL mice; Rg3 (25 μM) exerted similar effects in Ang II-treated MCFs. Meanwhile, Rg3 treatment ameliorated cardiac function and pathological features, and it also attenuated myocardial fibrosis in vivo and in vitro. In Ang II-treated MCFs, the effects of Rg3 on collagen deposition and TGF-β1/Smad3 pathway were slightly enhanced by overexpression of ACY1, whereas ACY1 siRNA partially weakened the beneficial effects of Rg3, suggesting that Rg3 might suppress myocardial fibrosis through ACY1. Our study demonstrates that N-acetylglutamine may be a potential biomarker of HF and its specific metabolic-degrading enzyme ACY1 could be a potential therapeutic target for the prevention and treatment of myocardial fibrosis during the development of HF. Rg3 attenuates myocardial fibrosis to ameliorate HF through increasing ACY1 expression and inhibiting TGF-β1/Smad3 pathway, which provides some references for further development of anti-fibrotic drugs for HF.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Serenelli M, Jackson A, Dewan P, Jhund PS, Petrie MC, Rossignol P, et al. Mineralocorticoid receptor antagonists, blood pressure, and outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8:188–98.
González A, Schelbert EB, Díez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71:1696–706.
Truby LK, Rogers JG. Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. 2020;8:523–36.
Wintrich J, Kindermann I, Ukena C, Selejan S, Werner C, Maack C, et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin Res Cardiol. 2020;109:1079–98.
Heymans S, González A, Pizard A, Papageorgiou AP, López-Andrés N, Jaisser F, et al. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail. 2015;17:764–71.
Gyöngyösi M, Winkler J, Ramos I, Do QT, Firat H, McDonald K, et al. Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail. 2017;19:177–91.
Guo Y, Gupte M, Umbarkar P, Singh AP, Sui JY, Force T, et al. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J Mol Cell Cardiol. 2017;110:109–20.
Rodriguez P, Sassi Y, Troncone L, Benard L, Ishikawa K, Gordon RE, et al. Deletion of delta-like 1 homologue accelerates fibroblast-myofibroblast differentiation and induces myocardial fibrosis. Eur Heart J. 2019;40:967–78.
Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19:57–75.
Tallquist MD. Cardiac fibroblast diversity. Annu Rev Physiol. 2020;82:63–78.
Bajaj JS, Reddy KR, O’Leary JG, Vargas HE, Lai JC, Kamath PS, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute-on-chronic liver failure and death in patients with cirrhosis. Gastroenterology. 2020;159:1715–30.
Figlia G, Willnow P, Teleman AA. Metabolites regulate cell signaling and growth via covalent modification of proteins. Dev Cell. 2020;54:156–70.
Karmazyn M, Gan XT. Treatment of the cardiac hypertrophic response and heart failure with ginseng, ginsenosides, and ginseng-related products. Can J Physiol Pharmacol. 2017;95:1170–6.
Li S, Yu Y, Bian X, Yao L, Li M, Lou YR, et al. Prediction of oral hepatotoxic dose of natural products derived from traditional Chinese medicines based on SVM classifier and PBPK modeling. Arch Toxicol 2021;95:1683–701.
Wu W, Jiao C, Li H, Ma Y, Jiao L, Liu S. LC-MS based metabolic and metabonomic studies of Panax ginseng. Phytochem Anal. 2018;29:331–40.
Kim JH. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42:264–9.
Shi ZY, Zeng JZ, Wong AST. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019;24:2443.
Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang S, et al. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis. 2020;11:454.
Tang M, Bian W, Cheng L, Zhang L, Jin R, Wang W, et al. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGF-β/Smad and ERK signaling pathways. Int J Mol Med. 2018;41:1487–99.
Wang M, Chen L, Liu D, Chen H, Tang DD, Zhao YY. Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem Biol Interact. 2017;273:133–41.
Wang Y, Wang J, Yao M, Zhao X, Fritsche J, Schmitt-Kopplin P, et al. Metabonomics study on the effects of the ginsenoside Rg3 in a beta-cyclodextrin-based formulation on tumor-bearing rats by a fully automatic hydrophilic interaction/reversed-phase column-switching HPLC-ESI-MS approach. Anal Chem. 2008;80:4680–8.
Miao H, Cao G, Wu XQ, Chen YY, Chen DQ, Chen L, et al. Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177:3415–35.
Chen DQ, Cao G, Chen H, Argyopoulos CP, Yu H, Su W, et al. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat Commun. 2019;10:1476.
Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, et al. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;76:4961–78.
Wang X, Zhang A, Yan G, Sun W, Han Y, Sun H. Metabolomics and proteomics annotate therapeutic properties of geniposide: targeting and regulating multiple perturbed pathways. PLoS One. 2013;8:e71403.
Gu Y, Zhang Y, Shi X, Li X, Hong J, Chen J, et al. Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta. 2010;81:766–72.
Lai Q. Study on the dynamic syndrome transformation characteristics of “from deficiency to stasis” and the characteristics of traditional Chinese medicine prescription and syndrome [D]. China Pharmaceutical University, 2020.
Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–53.
Morris J, Dunham A. Metoprolol. In: StatPearls [Internet]. Treasure Island (FL): (StatPearls Publishing; 2021) PMID: 30422518.
Lobo-Gonzalez M, Galán-Arriola C, Rossello X, González-Del-Hoyo M, Vilchez JP, Higuero-Verdejo MI, et al. Metoprolol blunts the time-dependent progression of infarct size. Basic Res Cardiol. 2020;115:55.
Qi J, Tan Y, Fan D, Pan W, Yu J, Xu W, et al. Songling Xuemaikang Capsule inhibits isoproterenol-induced cardiac hypertrophy via CaMKIIδ and ERK1/2 pathways. J Ethnopharmacol. 2020;253:112660.
Lorca R, Jiménez-Blanco M, García-Ruiz JM, Pizarro G, Fernández-Jiménez R, García-Álvarez A, et al. Coexistence of transmural and lateral wavefront progression of myocardial infarction in the human heart. Rev Esp Cardiol (Engl Ed). 2021;74:870–7.
Lai Q, Yuan G, Shen L, Zhang L, Fu F, Liu Z, et al. Oxoeicosanoid receptor inhibition alleviates acute myocardial infarction through activation of BCAT1. Basic Res Cardiol. 2021;116:3.
Ma D, Zheng B, Du H, Han X, Zhang X, Zhang J, et al. The mechanism underlying the protective effects of tannic acid against isoproterenol-induced myocardial fibrosis in mice. Front Pharmacol. 2020;11:716.
Fan Q, Tao R, Zhang H, Xie H, Lu L, Wang T, et al. Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation. 2019;139:663–78.
Lang M, Ou D, Liu Z, Li Y, Zhang X, Zhang F. LncRNA MHRT promotes cardiac fibrosis via miR-3185 pathway following myocardial infarction. Int Heart J. 2021;62:891–9.
Zhang QJ, He Y, Li Y, Shen H, Lin L, Zhu M, et al. Matricellular protein Cilp1 promotes myocardial fibrosis in response to myocardial infarction. Circ Res. 2021;129:1021–1035.
Lai Q, Yuan GY, Wang H, Liu ZL, Kou JP, Yu BY, et al. Exploring the protective effects of schizandrol A in acute myocardial ischemia mice by comprehensive metabolomics profiling integrated with molecular mechanism studies. Acta Pharmacol Sin. 2020;41:1058–72.
Zhou K, Ding X, Yang J, Hu Y, Song Y, Chen M, et al. Metabolomics reveals metabolic changes caused by low-dose 4-Tert-Octylphenol in mice liver. Int J Environ Res Public Health. 2018;15:2686.
Carrola J, Rocha CM, Barros AS, Gil AM, Goodfellow BJ, Carreira IM, et al. Metabolic signatures of lung cancer in biofluids: NMR-based metabonomics of urine. J Proteome Res. 2011;10:221–30.
Bradford BU, O’Connell TM, Han J, Kosyk O, Shymonyak S, Ross PK, et al. Metabolomic profiling of a modified alcohol liquid diet model for liver injury in the mouse uncovers new markers of disease. Toxicol Appl Pharmacol. 2008;232:236–43.
Sass JO, Mohr V, Olbrich H, Engelke U, Horvath J, Fliegauf M, et al. Mutations in ACY1, the gene encoding aminoacylase 1, cause a novel inborn error of metabolism. Am J Hum Genet. 2006;78:401–9.
Yu B, Liu X, Cao X, Zhang M, Chang H. Study of the expression and function of ACY1 in patients with colorectal cancer. Oncol Lett. 2017;13:2459–64.
Shi H, Hayes MT, Kirana C, Miller RJ, Keating JP, Stubbs RS. Overexpression of aminoacylase 1 is associated with colorectal cancer progression. Hum Pathol. 2013;44:1089–97.
Wei X, Li J, Xie H, Ling Q, Wang J, Lu D, et al. Proteomics-based identification of the tumor suppressor role of aminoacylase 1 in hepatocellular carcinoma. Cancer Lett. 2014;351:117–25.
Zhong Y, Onuki J, Yamasaki T, Ogawa O, Akatsuka S, Toyokuni S. Genome-wide analysis identifies a tumor suppressor role for aminoacylase 1 in iron-induced rat renal cell carcinoma. Carcinogenesis. 2009;30:158–64.
Fujioka T, Kühn A, Sanchez-Martinez S, Bijnens BH, Hui W, Slorach C, et al. Impact of interventricular interactions on left ventricular function, stroke volume, and exercise capacity in children and adults with ebstein’s anomaly. JACC Cardiovasc Imaging. 2019;12:925–7.
Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:782–94.
Cunningham JW, Claggett BL, O’Meara E, Prescott MF, Pfeffer MA, Shah SJ, et al. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol. 2020;76:503–14.
McLaughlin S, McNeill B, Podrebarac J, Hosoyama K, Sedlakova V, Cron G, et al. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat Commun. 2019;10:4866.
Rusu M, Hilse K, Schuh A, Martin L, Slabu I, Stoppe C, et al. Biomechanical assessment of remote and postinfarction scar remodeling following myocardial infarction. Sci Rep. 2019;9:16744.
Friebel J, Weithauser A, Witkowski M, Rauch BH, Savvatis K, Dörner A, et al. Protease-activated receptor 2 deficiency mediates cardiac fibrosis and diastolic dysfunction. Eur Heart J. 2019;40:3318–32.
Trippel TD, Van Linthout S, Westermann D, Lindhorst R, Sandek A, Ernst S, et al. Investigating a biomarker-driven approach to target collagen turnover in diabetic heart failure with preserved ejection fraction patients. Effect of torasemide versus furosemide on serum C-terminal propeptide of procollagen type I (DROP-PIP trial). Eur J Heart Fail. 2018;20:460–70.
Chiang MH, Liang CJ, Lin LC, Yang YF, Huang CC, Chen YH, et al. miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J Cell Physiol. 2020;235:6085–102.
Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–88.
Miller YE, Minna JD, Gazdar AF. Lack of expression of aminoacylase-1 in small cell lung cancer. Evidence for inactivation of genes encoded by chromosome 3p. J Clin Invest. 1989;83:2120–4.
Oatmen KE, Cull E, Spinale FG. Heart failure as interstitial cancer: emergence of a malignant fibroblast phenotype. Nat Rev Cardiol. 2020;17:523–31.
Ren B, Feng J, Yang N, Guo Y, Chen C, Qin Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int Immunopharmacol. 2021;98:107841.
Titus VH, Cowling AS, Kailasam RT. S. Collagen receptor cross-talk determines α-smooth muscle actin-dependent collagen gene expression in angiotensin II-stimulated cardiac fibroblasts. J Biol Chem. 2019;294:19723–39.
Zile MR, O’Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73:795–806.
Acknowledgements
This research work was supported by the National Natural Science Foundation of China (81774150, 81973506, 82104437), Natural Science Foundation of Jiangsu Province (BK20210431), China Postdoctoral Science Foundation (2021M693519), National Innovation and Entrepreneurship Training Program for Undergraduate (202110316013Y) and “Double First-Class” University project (CPU2018GF06, CPU2018GF07).
Author information
Authors and Affiliations
Contributions
BYY and FL designed research; QL, FML, and WLR performed research; GYY, ZYF, and LZ contributed new reagents or analytic tools; FF and JPK analyzed data; FL and QL wrote the paper. All the authors have approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Lai, Q., Liu, Fm., Rao, Wl. et al. Aminoacylase-1 plays a key role in myocardial fibrosis and the therapeutic effects of 20(S)-ginsenoside Rg3 in mouse heart failure. Acta Pharmacol Sin 43, 2003–2015 (2022). https://doi.org/10.1038/s41401-021-00830-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00830-1
Keywords
This article is cited by
-
Natural anti-cancer products: insights from herbal medicine
Chinese Medicine (2025)
-
Miglustat ameliorates isoproterenol-induced cardiac fibrosis via targeting UGCG
Molecular Medicine (2025)
-
Modulation of metabolic, inflammatory and fibrotic pathways by semaglutide in metabolic dysfunction-associated steatohepatitis
Nature Medicine (2025)
-
Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway
BMC Complementary Medicine and Therapies (2024)
-
Acetylglutamine Differentially Associated with First-Time Versus Recurrent Stroke
Translational Stroke Research (2024)