Abstract
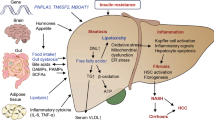
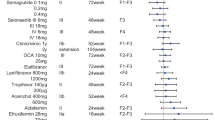
Nonalcoholic fatty liver disease is a growing public health crisis, with phenotypes from nonalcoholic fatty liver to nonalcoholic steatohepatitis, currently known as NASH, which can progress to liver fibrosis and end stage cirrhosis. NASH is associated with an increased risk of cardiovascular disease and Type 2 diabetes mellitus. There are still no U.S. FDA approved drugs or biological treatments for NASH or related liver diseases. Despite official agency guidance, the regulatory pathway to ultimate product approval is unclear, due to both the extra-hepatic factors that contribute to NASH, as well as the organizational structure of FDA, with its traditional separation of therapeutic indications within discrete review divisions. There is hope that continued evolution of the regulatory process will lead to the ability for clinical trial endpoints supporting NASH treatment approval to include both liver-based and traditional metabolic measures, independent of specific FDA division review.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ratziua V, Bellentanib S, Cortez-Pintoc H, Dayd C, Marchesinie G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84. http://www.journal-of-hepatology.eu/article/S0168-8278(10)00414-9/fulltext.
Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. https://doi.org/10.1002/hep.21327/abstract;jsessionid=05BED8DE29EA91ADB17E9A321FEB71FC.f04t01.
Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. https://www.ncbi.nlm.nih.gov/pubmed/20879883.
Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do Diabetologists stand? Clin Diabetes Endocrinol. 2020;6:9 https://doi.org/10.1186/s40842-020-00097-1.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. https://doi.org/10.1002/hep.29367
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. http://www.nejm.org/doi/abstract/10.1056/NEJMra1503519
U.S. Food & Drug Administration: News & Events. http://www.fda.gov/news-events/.
FDA Guidance Drug-Induced Liver Injury: Premarketing Clinical Evaluation July 2009. https://www.fda.gov/media/116737/download.
Sanyal A, Brunt EM, Kleiner DE, Kowdley K, Chalasani N, Lavine J, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–53. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4014460/.
Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry (December 2018) Draft Guidance. https://www.fda.gov/media/119044/download.
Nonalcoholic Steatohepatitis with Compensated Cirrhosis: Developing Drugs for Treatment Guidance for Industry (June 2019) Draft Guidance https://www.fda.gov/media/127738/download.
Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment (REGENERATE) NCT02548351. https://clinicaltrials.gov/ct2/show/NCT02548351.
Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, et al. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials. 2019;84:105803 https://doi.org/10.1016/j.cct.2019.06.017.
Intercept Pharmaceuticals, Inc. Press Release June 29, 2020. https://ir.interceptpharma.com/news-releases/news-release-details/intercept-receives-complete-response-letter-fda-obeticholic-acid
Anania FA, Dimick-Santos L, Mehta R, Toerner J, Beitz J. Nonalcoholic steatohepatitis: current thinking from the division of hepatology and nutrition at the food and drug administration. Hepatology. 2020;73:2023–7. https://doi.org/10.1002/hep.31687.
Regulatory Perspectives for Development of Drugs for Treatment of NASH - 01/29/2021 - 01/29/2021 | FDA. www.fda.gov/drugs/news.
Guidance for Industry Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes (December 2008). https://www.fda.gov/media/71297/download
Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control Guidance for Industry (March 2020). (https://www.fda.gov/media/135936/download.
Penson PE, Pirro M, Banach M. LDL-C: lower is better for longer—even at low risk. BMC Med. 2020;18:320–5. https://doi.org/10.1186/s12916-020-01792-7.
Reorganization of the Office of New Drugs with Corresponding Changes to the Office of Translational Sciences and the Office of Pharmaceutical Quality https://www.fda.gov/drugs/regulatory-science-research-and-education/reorganization-office-new-drugs-corresponding-changes-office-translational-sciences-and-office.
Office of Immunology and Inflammation - Division of Hepatology and Nutrition (DHN). https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-immunology-and-inflammation-division-hepatology-and-nutrition-dhn.
Division of Cardiology and Nephrology. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-cardiology-hematology-endocrinology-and-nephrology-ochen-division-cardiology-and-nephrology.
Division of Diabetes, Lipid Disorders, and Obesity. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-cardiology-hematology-endocrinology-and-nephrology-division-diabetes-lipid-disorders-and.
JARDIANCE® (empagliflozin tablets) Label Revision August 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/204629s026lbl.org.
FDA Jardiance Supplement Approval Letter (August 18, 2021) Norman Stockbridge. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/204629Orig1s026ltr.pdf.
Patient-focused drug development (PFDD). https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development.
U.S. Preventive Services Task Force https://www.uspreventiveservicestaskforce.org/uspstf/.
Screening for Prediabetes and Type 2 Diabetes US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736–43. https://jamanetwork.com/journals/jama/fullarticle/2783414.
Screening for Prediabetes and Type 2 Diabetes August 24, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes.
Global Liver Institute. https://www.globalliver.org/about.
USPSTF Must Include Link to Liver Disease in Recommendation on Screening for Diabetes. https://www.globalliver.org/news/2021/04/uspstf-must-include-link-to-liver-disease-in-recommendation-on-screening-for-diabetes.
Letter To USPSTF April 9, 2021. https://static1.squarespace.com/static/53bafd3ce4b0ae714af7153f/t/60759994a5a7834044a70c8b/1618319765042/Response+to+USPSTF+RE_+Diabetes+and+Prediabetes+Screening.pdf.
Patient-Driven Transformation is Key to NASH Clinical Trials August 25, 2021. https://www.globalliver.org/news/2021/nash-news-august.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Rights and permissions
About this article
Cite this article
Harvey, B.E. NASH: regulatory considerations for clinical drug development and U.S. FDA approval. Acta Pharmacol Sin 43, 1210–1214 (2022). https://doi.org/10.1038/s41401-021-00832-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00832-z
Keywords
This article is cited by
-
How improvements in US FDA regulatory process and procedures led to the drug approval for first ever treatment of a common liver disease
Acta Pharmacologica Sinica (2025)
-
Rotundic acid improves nonalcoholic steatohepatitis in mice by regulating glycolysis and the TLR4/AP1 signaling pathway
Lipids in Health and Disease (2023)
-
iTRAQ-based quantitative proteomics analysis of the effect of ACT001 on non-alcoholic steatohepatitis in mice
Scientific Reports (2023)
-
Age-related liver endothelial zonation triggers steatohepatitis by inactivating pericentral endothelium-derived C-kit
Nature Aging (2022)
-
All about NASH: disease biology, targets, and opportunities on the road to NASH drugs
Acta Pharmacologica Sinica (2022)