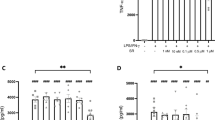
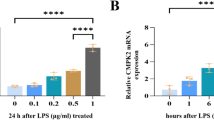
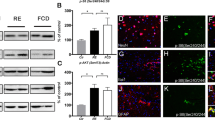
Abstract
Neuroinflammation is closely related to the pathogenesis of neurodegenerative diseases. Activation of microglia, the resident immune cells in CNS, induces inflammatory responses, resulting in the release of neurotoxic molecules, which favors neuronal death and neurodegeneration. Nuclear receptor-related 1 (Nurr1) protein, one of the orphan nuclear receptor superfamilies, is an emerging target for neuroprotective therapy. In addition, the anti-inflammatory function of cannabinoid (CB) receptors has attracted increasing interest. As both CB receptors (especially CB2 receptor) and Nurr1 exist in microglia, and regulate a number of same molecular points such as NF-κB, we herein explored the interplay between the CB2 receptor and Nurr1 as well as the regulatory mechanisms in microglial cells. We showed that the application of CB2 receptor agonists JWH015 (1, 10 μM) significantly increased the nuclear Nurr1 protein in BV-2 cells and primary midbrain microglia. Overexpression of Nurr1 or application of Nurr1 agonist C-DIM12 (10 μM) significantly increased the mRNA level of CB2 receptor in BV-2 cells, suggesting that positive expression feedback existing between the CB2 receptor and Nurr1. After 2-AG and JWH015 activated the CB2 receptors, the levels of p-ERK, p-AKT, p-GSK-3β in BV-2 cells were significantly increased. Using ERK1/2 inhibitor U0126 and PI3K/AKT inhibitor LY294002, we revealed that the amount of Nurr1 in the nucleus was upregulated through β-arrestin2/ERK1/2 and PI3K/AKT/GSK-3β signaling pathways. With these inhibitors, we found a cross-talk interaction between the two pathways, and the ERK1/2 signaling pathway played a more dominant regulatory role. Furthermore, we demonstrated that when the CB2 receptor was activated, the phagocytic function of BV-2 cells was significantly weakened; the activation of Nurr1 also inhibited the phagocytic function of BV-2 cells. Pretreatment with the signaling pathway inhibitors, especially U0126, reversed the inhibitory effect of 2-AG on phagocytosis, suggesting that CB2 receptor may regulate the phagocytic function of microglia by activating Nurr1. In conclusion, CB2 receptor or/and Nurr1-mediated signal pathways play instrumental roles in the progress of phagocytosis, which are expected to open up new treatment strategies for neurodegenerative diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
18 June 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41401-024-01337-1
References
Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron. 2002;35:419–32.
Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25.
Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–39.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34.
Kopitar-Jerala N. Innate immune response in brain, NF-kappa B signaling, and cystatins. Front Mol Neurosci. 2015;8:73.
Lu H-C, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–25.
Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833.
Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56:244–53.
Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 2005:299–325. https://doi.org/10.1007/3-540-26573-2_10.
Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond Ser B: Biol Sci. 2001;356:381–408.
Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–30.
Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol. 2007;5:73–80.
Liu Z, Wang Y, Zhao H, Zheng Q, Xiao L, Zhao M. CB2 receptor activation ameliorates the proinflammatory activity in acute lung injury induced by paraquat. Biomed Res Int. 2014;2014:971750.
Zhu M, Yu B, Bai J, Wang X, Guo X, Liu Y, et al. Cannabinoid receptor 2 agonist prevents local and systemic inflammatory bone destruction in rheumatoid arthritis. J Bone Min Res. 2019;34:739–51.
Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017;11:30.
Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog Neurobiol. 2005;77:128–38.
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59.
Liu W, Gao Y, Chang N. Nurr1 overexpression exerts neuroprotective and anti-inflammatory roles via down-regulating CCL2 expression in both in vivo and in vitro Parkinson’s disease models. Biochem Biophys Res Commun. 2017;482:1312–9.
Bensinger SJ, Tontonoz P. A Nurr1 pathway for neuroprotection. Cell. 2009;137:26–8.
Kim C-H, Han B-S, Moon J, Kim D-J, Shin J, Rajan S, et al. Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc Natl Acad Sci USA. 2015;112:8756–61.
Sim Y, Park G, Eo H, Huh E, Gu PS, Hong S-P, et al. Protective effects of a herbal extract combination of Bupleurum falcatum, Paeonia suffruticosa, and Angelica dahurica against MPTP-induced neurotoxicity via regulation of nuclear receptor-related 1 protein. Neuroscience. 2017;340:166–75.
Shao Q-H, Yan W-F, Zhang Z, Ma K-L, Peng S-Y, Cao Y-L, et al. Nurr1: A vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology. 2019;144:388–99.
Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor*. J Biol Chem. 2006;281:1261–73.
Merighi S, Gessi S, Varani K, Simioni C, Fazzi D, Mirandola P, et al. Cannabinoid CB(2) receptors modulate ERK-1/2 kinase signalling and NO release in microglial cells stimulated with bacterial lipopolysaccharide. Br J Pharmacol. 2012;165:1773–88.
Zhang T, Jia N, Fei E, Wang P, Liao Z, Ding L, et al. Nurr1 is phosphorylated by ERK2 in vitro and its phosphorylation upregulates tyrosine hydroxylase expression in SH-SY5Y cells. Neurosci Lett. 2007;423:118–22.
Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharm Toxicol. 1998;38:179–200.
Fechtner S, Singh AK, Srivastava I, Szlenk CT, Muench TR, Natesan S, et al. Cannabinoid receptor 2 agonist JWH-015 inhibits interleukin-1β-induced inflammation in rheumatoid arthritis synovial fibroblasts and in adjuvant induced arthritis rat via glucocorticoid receptor. Front Immunol. 2019;10:1027.
Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564.
Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–14.
De Miranda BR, Popichak KA, Hammond SL, Jorgensen BA, Phillips AT, Safe S, et al. The Nurr1 activator 1,1-bis(3’-Indolyl)-1-(p-chlorophenyl)methane blocks inflammatory gene expression in BV-2 microglial cells by inhibiting nuclear factor κB. Mol Pharmacol. 2015;87:1021–34.
Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010;31:24–31.
Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965−1014.
Su H-C, Ma C-T, Yu B-C, Chien Y-C, Tsai C-C, Huang W-C, et al. Glycogen synthase kinase-3β regulates anti-inflammatory property of fluoxetine. Int Immunopharmacol. 2012;14:150–6.
De Virgilio A, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A, et al. Parkinson’s disease: autoimmunity and neuroinflammation. Autoimmun Rev. 2016;15:1005–11.
Neher JJ, Neniskyte U, Zhao J-W, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973.
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69.
Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain. 2009;132:288–95.
Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6.
Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808.
Vilalta A, Brown GC. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2018;285:3566–75.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8.
Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1628:288–97.
Emmrich JV, Hornik TC, Neher JJ, Brown GC. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013;280:5030–8.
Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–16.
Butler CA, Popescu AS, Kitchener EJA, Allendorf DH, Puigdellívol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021;158:621–39.
Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: Transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol. 2010;30:1535–41.
Le W, Wu J, Tang Y. Protective microglia and their regulation in Parkinson’s disease. Front Mol Neurosci. 2016;9:89.
Chen H, Yu X, Hu L, Peng Y, Yu Q, Zhou H, et al. Activation of Nurr1 with amodiaquine protected neuron and alleviated neuroinflammation after subarachnoid hemorrhage in rats. Oxid Med Cell Longev. 2021;2021:6669787.
Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–45.
Tanaka M, Sackett S, Zhang Y. Endocannabinoid modulation of microglial phenotypes in neuropathology. Front Neurol. 2020;11:87.
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29.
Guida F, Luongo L, Boccella S, Giordano ME, Romano R, Bellini G, et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the CB2 receptor. Sci Rep. 2017;7:375.
Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12.
Hytti M, Andjelic S, Josifovska N, Piippo N, Korhonen E, Hawlina M, et al. CB2 receptor activation causes an ERK1/2-dependent inflammatory response in human RPE cells. Sci Rep. 2017;7:16169.
Ofek O, Attar-Namdar M, Kram V, Dvir-Ginzberg M, Mechoulam R, Zimmer A, et al. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J Bone Min Res. 2011;26:308–16.
Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9.
Fan X, Luo G, Ming M, Pu P, Li L, Yang D, et al. Nurr1 expression and its modulation in microglia. Neuroimmunomodulation. 2009;16:162–70.
Gokoh M, Kishimoto S, Oka S, Sugiura T. 2-Arachidonoylglycerol enhances the phagocytosis of opsonized zymosan by HL-60 cells differentiated into macrophage-like cells. Biol Pharm Bull. 2007;30:1199–205.
Tolón RM, Núñez E, Pazos MR, Benito C, Castillo AI, Martínez-Orgado JA, et al. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. 2009;1283:148–54.
Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–21.
Sánchez MG, Ruiz-Llorente L, Sánchez AM, Díaz-Laviada I. Activation of phosphoinositide 3-kinase/PKB pathway by CB1 and CB2 cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal. 2003;15:851–9.
Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623.
Suchard SJ, Mansfield PJ, Boxer LA, Shayman JA. Mitogen-activated protein kinase activation during IgG-dependent phagocytosis in human neutrophils: Inhibition by ceramide. J Immunol. 1997;158:4961.
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8.
Rhim JH, Luo X, Gao D, Xu X, Zhou T, Li F, et al. Cell type-dependent Erk-Akt pathway crosstalk regulates the proliferation of fetal neural progenitor cells. Sci Rep. 2016;6:26547.
Gaikwad S, Larionov S, Wang Y, Dannenberg H, Matozaki T, Monsonego A, et al. Signal regulatory protein-β1: A microglial modulator of phagocytosis in Alzheimer’s disease. Am J Pathol. 2009;175:2528–39.
Ayata P, Badimon A, Strasburger HJ, Duff MK, Montgomery SE, Loh Y-HE, et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci. 2018;21:1049–60.
Carrillo-Jimenez A, Deniz Ö, Niklison-Chirou MV, Ruiz R, Bezerra-Salomao K, Stratoulias V, et al. TET2 regulates the neuroinflammatory response in microglia. Cell Rep. 2019;29:697–713. e8.
Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49:1422–34.
Reusch N, Ravichandran KA, Olabiyi BF, Komorowska-Muller JA, Hansen JN, Ulas T, et al. Cannabinoid receptor 2 is necessary to induce toll-like receptor-mediated microglial activation. Glia. 2022;70:71–88.
Acknowledgements
This work was supported by National Health Commission Key Laboratory of Drug Addiction Medicine, the First Affiliated Hospital of Kunming Medical University (Kunming, China, 2020DAMOP-008), the National Natural Science Foundation of China (81773925 and 82104418), the Beijing Natural Science Foundation (7212156), CAMS Innovation Fund for Medical Sciences (2021-I2M-1-026), and the Fundamental Research Funds for the Central Universities (3332019154 and 3332019153).
Author information
Authors and Affiliations
Contributions
YHY, NHC, and KLM conceived and designed the experiments; QWH and QHS performed and analyzed the data; XTW supplemented part of the experiments; QWH wrote the manuscript and prepared the figures; YHY, XTW, and QWH revised the paper; YHY and NHC interpreted the data. All authors reviewed and approved the final version of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
The original online version of this article was revised: Figures 8 c-d have been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, Qw., Shao, Qh., Wang, Xt. et al. CB2 receptor activation inhibits the phagocytic function of microglia through activating ERK/AKT-Nurr1 signal pathways. Acta Pharmacol Sin 43, 2253–2266 (2022). https://doi.org/10.1038/s41401-021-00853-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00853-8
Keywords
This article is cited by
-
Melatonin mitigates hormonal toxicity in cannabis-treated female Wistar rats: involvement of cannabinoid receptor
Journal of Cannabis Research (2026)
-
Targeting microglial NAAA-regulated PEA signaling counters inflammatory damage and symptom progression of post-stroke anxiety
Cell Communication and Signaling (2025)
-
Physical Exercise Improves the Neuronal Function in Ischemic Stroke Via Microglial CB2R/P2Y12 Signaling
Molecular Neurobiology (2025)
-
Complement Molecule C3a Exacerbates Early Brain Injury After Subarachnoid Hemorrhage by Inducing Neuroinflammation Through the C3aR-ERK-P2X7-NLRP3 Inflammasome Signaling Axis
Inflammation (2024)
-
The RNA m6A modification might participate in microglial activation during hypoxic–ischemic brain damage in neonatal mice
Human Genomics (2023)