Abstract
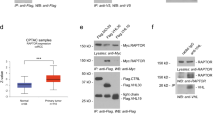
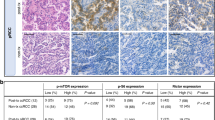
Rapalogs (everolimus and temsirolimus) are allosteric mTORC1 inhibitors and approved agents for advanced clear cell renal cell carcinoma (ccRCC), although only a subset of patients derive clinical benefit. Progress in genomic characterization has made it possible to generate comprehensive profiles of genetic alterations in ccRCC; however, the correlations between recurrent somatic mutations and rapalog efficacy remain unclear. Here, we demonstrate by using multiple patient-derived ccRCC cell lines that compared to PTEN-proficient cells, PTEN-deficient cells exhibit hypersensitivity to rapalogs. Rapalogs inhibit cell proliferation by inducing G0/G1 arrest without inducing apoptosis in PTEN-deficient ccRCC cell lines. Using isogenic cell lines generated by CRISPR/Cas9, we validate the correlation between PTEN loss and rapalog hypersensitivity. In contrast, deletion of VHL or chromatin-modifying genes (PBRM1, SETD2, BAP1, or KDM5C) fails to influence the cellular response to rapalogs. Our mechanistic study shows that ectopic expression of an activating mTOR mutant (C1483F) antagonizes PTEN-induced cell growth inhibition, while introduction of a resistant mTOR mutant (A2034V) enables PTEN-deficient ccRCC cells to escape the growth inhibitory effect of rapalogs, suggesting that PTEN loss generates vulnerability to mTOR inhibition. PTEN-deficient ccRCC cells are more sensitive to the inhibitory effects of temsirolimus on cell migration and tumor growth in zebrafish and xenograft mice, respectively. Of note, PTEN protein loss as detected by immunohistochemistry is much more frequent than mutations in the PTEN gene in ccRCC patients. Our study suggests that PTEN loss correlates with rapalog sensitivity and could be used as a marker for ccRCC patient selection for rapalog therapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The RNA-seq data have been deposited to the National Center for Biotechnology Information (NCBI) via the Sequence Read Archive (SRA) database repository under accession code PRJNA777372. All other relevant data supporting the findings of this study are available upon reasonable request.
References
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Prim. 2017;3:17009.
Xiao GF, Yan X, Chen Z, Zhang RJ, Liu TZ, Hu WL. Identification of a novel immune-related prognostic biomarker and small-molecule drugs in clear cell renal cell carcinoma (ccRCC) by a merged microarray-acquired dataset and TCGA database. Front Genet. 2020;11:810.
Dizman N, Philip EJ, Pal SK. Genomic profiling in renal cell carcinoma. Nat Rev Nephrol. 2020;16:435–51.
Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013; 499: 43-9.
Ho TH, Choueiri TK, Wang K, Karam JA, Chalmers Z, Frampton G, et al. Correlation between molecular subclassifications of clear cell renal cell carcinoma and targeted therapy response. Eur Urol Focus. 2016;2:204–9.
Kwiatkowski DJ, Choueiri TK, Fay AP, Rini BI, Thorner AR, de Velasco G, et al. Mutations in TSC1, TSC2, and MTOR Are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2016;22:2445–52.
Lei Y, Yildiz S, Chen M. Re: James J. Hsieh, David Chen, Patricia I. Wang, et al. Genomic biomarkers of a randomized trial comparing first-line everolimus and sunitinib in patients with metastatic renal cell carcinoma. Eur Urol. 2017;71:405–14.
Voss MH, Chen D, Reising A, Marker M, Shi J, Xu J, et al. PTEN Expression, not mutation status in TSC1, TSC2, or mTOR, correlates with the outcome on everolimus in patients with renal cell carcinoma treated on the randomized RECORD-3 trial. Clin Cancer Res. 2019;25:506–14.
Roldan-Romero JM, Beuselinck B, Santos M, Rodriguez-Moreno JF, Lanillos J, Calsina B, et al. PTEN expression and mutations in TSC1, TSC2 and MTOR are associated with response to rapalogs in patients with renal cell carcinoma. Int J Cancer. 2020;146:1435–44.
Nassar AH, Hamieh L, Gray KP, Thorner AR, Fay AP, Lasseter KD, et al. Mutations and response to rapalogs in patients with metastatic renal cell carcinoma. Mol Cancer Ther. 2020;19:690–6.
Yu L, Zhou D, Zhang G, Ren Z, Luo X, Liu P, et al. Co-occurrence of BAP1 and SF3B1 mutations in uveal melanoma induces cellular senescence. Mol Oncol. 2021; https://doi.org/10.1002/1878-0261.13128.
Xu YY, Ren ZL, Liu XL, Zhang GM, Huang SS, Shi WH, et al. BAP1 loss augments sensitivity to BET inhibitors in cancer cells. Acta Pharmacol Sin. 2021; https://doi.org/10.1038/s41401-021-00783-5.
van der Meer D, Barthorpe S, Yang W, Lightfoot H, Hall C, Gilbert J, et al. Cell model passports-a hub for clinical, genetic and functional datasets of preclinical cancer models. Nucleic Acids Res. 2019;47:D923–d9.
Stebbins CE, Kaelin WG Jr, Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science. 1999;284:455–61.
Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–55.
Nargund AM, Pham CG, Dong Y, Wang PI, Osmangeyoglu HU, Xie Y, et al. The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma. Cell Rep. 2017;18:2893–906.
Campagne A, Lee MK, Zielinski D, Michaud A, Le Corre S, Dingli F, et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat Commun. 2019;10:348.
Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19:547–62.
Grabiner BC, Nardi V, Birsoy K, Possemato R, Shen K, Sinha S, et al. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4:554–63.
Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–9.
Xu J, Pham CG, Albanese SK, Dong Y, Oyama T, Lee CH, et al. Mechanistically distinct cancer-associated mTOR activation clusters predict sensitivity to rapamycin. J Clin Invest. 2016;126:3526–40.
Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272–6.
Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–46.
Burow ME, Weldon CB, Melnik LI, Duong BN, Collins-Burow BM, Beckman BS, et al. PI3-K/AKT regulation of NF-kappaB signaling events in suppression of TNF-induced apoptosis. Biochem Biophys Res Commun. 2000;271:342–5.
Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. 2003;278:3694–704.
Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21:63–71.
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8.
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8.
Zong H, Yin B, Zhou H, Cai D, Ma B, Xiang Y. Inhibition of mTOR pathway attenuates migration and invasion of gallbladder cancer via EMT inhibition. Mol Biol Rep. 2014;41:4507–12.
Hamieh L, Choueiri TK, Ogórek B, Khabibullin D, Rosebrock D, Livitz D, et al. Mechanisms of acquired resistance to rapalogs in metastatic renal cell carcinoma. PLoS Genet. 2018;14:e1007679.
Hujber Z, Petővári G, Szoboszlai N, Dankó T, Nagy N, Kriston C, et al. Rapamycin (mTORC1 inhibitor) reduces the production of lactate and 2-hydroxyglutarate oncometabolites in IDH1 mutant fibrosarcoma cells. J Exp Clin Cancer Res. 2017;36:74.
Tuloup-Minguez V, Hamaï A, Greffard A, Nicolas V, Codogno P, Botti J. Autophagy modulates cell migration and β1 integrin membrane recycling. Cell Cycle. 2013;12:3317–28.
Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol Res. 2020;152:104609.
Ngeow J, Eng C. PTEN in hereditary and sporadic cancer. Cold Spring Harb Perspect Med. 2020;10:a036087.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81973357), Guangdong Basic and Applied Basic Research Foundation (2020A1515010027), Guangzhou Basic and Applied Basic Research Foundation (4001616289), Wu Jieping Medical Foundation (3206750.2020-10-120), Bethune Charitable Foundation Research Programme (B-19-H-20200622) and HUI LAN Public Welfare (2021-HLJJ0328). We thank Dr. Kun-Liang Guan (University of California, San Diego) for critically discussing and revising the manuscript.
Author information
Authors and Affiliations
Contributions
XLL and GMZ substantially contributed to designing the study, performing experiments, and analyzing the data. WHS, LXY, and SSH performed experiments and analyzed the data. ZLR analyzed the data. JJZ and SWL provided experimental resources. LY and YLL conceived and designed the study and experiments, assembled and interpreted the data, wrote and revised the manuscript, and approved the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Liu, Xl., Zhang, Gm., Huang, Ss. et al. PTEN loss confers sensitivity to rapalogs in clear cell renal cell carcinoma. Acta Pharmacol Sin 43, 2397–2409 (2022). https://doi.org/10.1038/s41401-022-00862-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00862-1