Abstract
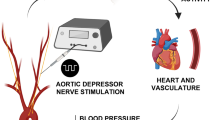
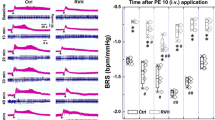
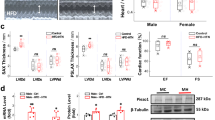
Recent studies suggest that melatonin (Mel) plays an important role in the regulation of blood pressure (BP) via the aortic baroreflex pathway. In this study, we investigated the interaction between the baroreflex afferent pathway and Mel-mediated BP regulation in rats under physiological and hypertensive conditions. Mel (0.1, 0.3, and 1.0 mg/mL) was microinjected into the nodose ganglia (NG) of rats. We showed that Mel-induced reduction of mean arterial pressure in female rats was significantly greater than that in male and in ovariectomized rats under physiological condition. Consistently, the expression of Mel receptors (MTNRs) in the NG of female rats was significantly higher than that of males. In L-NAME-induced hypertensive and spontaneously hypertensive rat models, MTNRs were upregulated in males but downregulated in female models. Interestingly, Mel-induced BP reduction was found in male hypertensive models. In whole-cell recording from identified baroreceptor neurons (BRNs) in female rats, we found that Mel (0.1 μM) significantly increased the excitability of a female-specific subpopulation of Ah-type BRNs by increasing the Nav1.9 current density via a PKC-mediated pathway. Similar results were observed in baroreceptive neurons of the nucleus tractus solitarius, showing the facilitation of spontaneous and evoked excitatory post-synaptic currents in Ah-type neurons. Collectively, this study reveals the estrogen-dependent effect of Mel/MTNRs under physiological and hypertensive conditions is mainly mediated by Ah-type BRNs, which may provide new theoretical basis and strategies for the gender-specific anti-hypertensive treatment in clinical practice.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017;34:10–22.
Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–6.
Simko F, Baka T, Krajcirovicova K, Repova K, Aziriova S, Zorad S, et al. Effect of melatonin on the renin-angiotensin-aldosterone system in L-NAME-induced hypertension. Molecules. 2018;23:265.
Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, et al. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006;119:898–902.
Grossman E, Laudon M, Zisapel N. Effect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trials. Vasc Health Risk Manag. 2011;7:577–84.
Smolensky MH, Hermida RC, Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med Rev. 2017;33:4–16.
Campos LA, Cipolla-Neto J, Michelini LC. Melatonin modulates baroreflex control via area postrema. Brain Behav. 2013;3:171–7.
Girouard H, Denault C, Chulak C, de Champlain J. Treatment by n-acetylcysteine and melatonin increases cardiac baroreflex and improves antioxidant reserve. Am J Hypertens. 2004;17:947–54.
Kitajima T, Kanbayashi T, Saitoh Y, Ogawa Y, Sugiyama T, Kaneko Y, et al. The effects of oral melatonin on the autonomic function in healthy subjects. Psychiatry Clin Neurosci. 2001;55:299–300.
Baker J, Kimpinski K. Role of melatonin in blood pressure regulation: an adjunct anti-hypertensive agent. Clin Exp Pharmacol Physiol. 2018;45:755–66.
Hildebrandt DA, Irwin ED, Lohmeier TE. Prolonged baroreflex activation abolishes salt-induced hypertension after reductions in kidney mass. Hypertension. 2016;68:1400–6.
Mar PL, Nwazue V, Black BK, Biaggioni I, Diedrich A, Paranjape SY, et al. Valsalva maneuver in pulmonary arterial hypertension: susceptibility to syncope and autonomic dysfunction. Chest. 2016;149:1252–60.
Shi Z, Li B, Brooks VL. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension. 2015;66:1034–41.
Li BY, Schild JH. Patch clamp electrophysiology in nodose ganglia of adult rat. J Neurosci Methods. 2002;115:157–67.
Li BY, Qiao GF, Feng B, Zhao RB, Lu YJ, Schild JH. Electrophysiological and neuroanatomical evidence of sexual dimorphism in aortic baroreceptor and vagal afferents in rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1301–10.
Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods. 2007;164:75–85.
Lu XL, Xu WX, Yan ZY, Qian Z, Xu B, Liu Y, et al. Subtype identification in acutely dissociated rat nodose ganglion neurons based on morphologic parameters. Int J Biol Sci. 2013;9:716–27.
Zhang YY, Yan ZY, Qu MY, Guo XJ, Li G, Lu XL, et al. KCa1.1 is potential marker for distinguishing Ah-type baroreceptor neurons in NTS and contributes to sex-specific presynaptic neurotransmission in baroreflex afferent pathway. Neurosci Lett. 2015;604:1–6.
Qiao GF, Li BY, Zhou YH, Lu YJ, Schild JH. Characterization of persistent TTX-R Na+ currents in physiological concentration of sodium in rat visceral afferents. Int J Biol Sci. 2009;5:293–7.
Huang J, Vanoye CG, Cutts A, Goldberg YP, Dib-Hajj SD, Cohen CJ, et al. Sodium channel NaV1.9 mutations associated with insensitivity to pain dampen neuronal excitability. J Clin Invest. 2017;127:2805–14.
Copel C, Osorio N, Crest M, Gola M, Delmas P, Clerc N. Activation of neurokinin 3 receptor increases Na(v)1.9 current in enteric neurons. J Physiol. 2009;587:1461–79.
Kurowski P, Grzelka K, Szulczyk P. Ionic mechanism underlying rebound depolarization in medial prefrontal cortex pyramidal neurons. Front Cell Neurosci. 2018;12:93.
Howard CM, Lutterschmidt DI. The effects of melatonin on brain arginine vasotocin: relationship with sex and seasonal differences in melatonin receptor type 1 in green treefrogs (Hyla cinerea). J Neuroendocrinol. 2015;27:670–9.
Viswanathan M, Laitinen JT, Saavedra JM. Differential expression of melatonin receptors in spontaneously hypertensive rats. Neuroendocrinology. 1992;56:864–70.
Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci USA. 2016;113:E2730–9.
Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Increased melatonin levels after hemorrhagic shock in male and female C3H/HeN mice. Experientia. 1996;52:587–90.
Sato S, Yin C, Teramoto A, Sakuma Y, Kato M. Sexually dimorphic modulation of GABA(A) receptor currents by melatonin in rat gonadotropin-releasing hormone neurons. J Physiol Sci. 2008;58:317–22.
Liu Y, Zhou JY, Zhou YH, Wu D, He JL, Han LM, et al. Unique expression of angiotensin type-2 receptor in sex-specific distribution of myelinated Ah-type baroreceptor neuron contributing to sex-dimorphic neurocontrol of circulation. Hypertension. 2016;67:783–91.
Qiao GF, Li BY, Lu YJ, Fu YL, Schild JH. 17Beta-estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am J Physiol Cell Physiol. 2009;297:C654–64.
Qiao GF, Qian Z, Sun HL, Xu WX, Yan ZY, Liu Y, et al. Remodeling of hyperpolarization-activated current, Ih, in Ah-type visceral ganglion neurons following ovariectomy in adult rats. PLoS One. 2013;8:e71184.
Wang LQ, Liu SZ, Wen X, Wu D, Yin L, Fan Y, et al. Ketamine-mediated afferent-specific presynaptic transmission blocks in low-threshold and sex-specific subpopulation of myelinated Ah-type baroreceptor neurons of rats. Oncotarget. 2015;6:44108–22.
Xu WX, Yu JL, Feng Y, Yan QX, Li XY, Li Y, et al. Spontaneous activities in baroreflex afferent pathway contribute dominant role in parasympathetic neurocontrol of blood pressure regulation. CNS Neurosci Ther. 2018;24:1219–30.
Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23:3908–15.
Liu Y, Zhao SY, Feng Y, Sun J, Lu XL, Yan QX, et al. Contribution of baroreflex afferent pathway to NPY-mediated regulation of blood pressure in rats. Neurosci Bull. 2020;36:396–406.
Liu Y, Wu D, Qu MY, He JL, Yuan M, Zhao M, et al. Neuropeptide Y-mediated sex- and afferent-specific neurotransmissions contribute to sexual dimorphism of baroreflex afferent function. Oncotarget. 2016;7:66135–48.
Sun J, He C, Yan QX, Wang HD, Li KX, Sun X, et al. Parkinson-like early autonomic dysfunction induced by vagal application of DOPAL in rats. CNS Neurosci Ther. 2021;27:540–51.
Liu Y, Wen X, Liu SZ, Song DX, Wu D, Guan J, et al. KCa1.1-mediated frequency-dependent central and peripheral neuromodulation via Ah-type baroreceptor neurons located within nodose ganglia and nucleus of solitary tract of female rats. Int J Cardiol. 2015;185:84–7.
Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–17.
Li B, Schild JH. Persistent tetrodotoxin-resistant Na+ currents are activated by prostaglandin E2 via cyclic AMP-dependent pathway in C-type nodose neurons of adult rats. Biochem Biophys Res Commun. 2007;355:1064–8.
Bai Q, Shao J, Cao J, Ren X, Cai W, Su S, et al. Protein kinase C-alpha upregulates sodium channel Nav1.9 in nociceptive dorsal root ganglion neurons in an inflammatory arthritis pain model of rat. J Cell Biochem. 2020;121:768–78.
Xu F, Zhong JY, Lin X, Shan SK, Guo B, Zheng MH, et al. Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J Pineal Res. 2020;68:e12631.
Regrigny O, Delagrange P, Scalbert E, Lartaud-Idjouadiene I, Atkinson J, Chillon JM. Effects of melatonin on rat pial arteriolar diameter in vivo. Br J Pharmaol. 1999;127:1666–70.
Zhang Y, Li H, Pu Y, Gong S, Liu C, Jiang X, et al. Melatonin-mediated inhibition of Purkinje neuron P-type Ca2+ channels in vitro induces neuronal hyperexcitability through the phosphatidylinositol 3-kinase-dependent protein kinase C delta pathway. J Pineal Res. 2015;58:321–34.
Kawashima K, Nagakura A, Wurzburger RJ, Spector S. Melatonin in serum and the pineal of spontaneously hypertensive rats. Clin Exp Hypertens A. 1984;6:1517–28.
Cagnacci A, Cannoletta M, Renzi A, Baldassari F, Arangino S, Volpe A. Prolonged melatonin administration decreases nocturnal blood pressure in women. Am J Hypertens. 2005;18:1614–8.
Simko F, Paulis L. Melatonin as a potential antihypertensive treatment. J Pineal Res. 2007;42:319–22.
Browning C, Beresford I, Fraser N, Giles H. Pharmacological characterization of human recombinant melatonin mt(1) and MT(2) receptors. Br J Pharmacol. 2000;129:877–86.
Nishi EE, Almeida VR, Amaral FG, Simon KA, Futuro-Neto HA, Pontes RB, et al. Melatonin attenuates renal sympathetic overactivity and reactive oxygen species in the brain in neurogenic hypertension. Hypertens Res. 2019;42:1683–91.
Li JN, Li XL, He J, Wang JX, Zhao M, Liang XB, et al. Sex- and afferent-specific differences in histamine receptor expression in vagal afferents of rats: a potential mechanism for sexual dimorphism in prevalence and severity of asthma. Neuroscience. 2015;303:166–77.
Wang LQ, Qian Z, Ma HL, Zhou M, Li HD, Cui CP, et al. Estrogen-dependent KCa1.1 modulation is essential for retaining neuroexcitation of female-specific subpopulation of myelinated Ah-type baroreceptor neurons in rats. Acta Pharmacol Sin. 2021;42:2173–80.
Li BY, Feng B, Tsu HY, Schild JH. Unmyelinated visceral afferents exhibit frequency dependent action potential broadening while myelinated visceral afferents do not. Neurosci Lett. 2007;421:62–6.
Li BY, Glazebrook P, Kunze DL, Schild JH. KCa1.1 channel contributes to cell excitability in unmyelinated but not myelinated rat vagal afferents [Research Support, N.I.H., Extramural]. Am J Physiol Cell Physiol. 2011;300:C1393–403.
Xu Z, Wu Y, Zhang Y, Zhang H, Shi L. Melatonin activates BKCa channels in cerebral artery myocytes via both direct and MT receptor/PKC-mediated pathway. Eur J Pharmacol. 2018;842:177–88.
Acknowledgements
This study was supported by research grants from the National Natural Science Foundation of China (81573431, 81971326, and 31871175). This work was especially dedicated to and commemorated for Professor/Dr. Wen-fen Chu for his outstanding performance and unselfish contribution to the Basic Medical Science as well as his personal character during his professional career at the Department of Pharmacology, Harbin Medical University. These authors would like also thank Dr. Rong Huo and Dr. Ning Wang for their helps and valuable inputs in qRT-PCR techniques and data interpretations.
Author information
Authors and Affiliations
Contributions
DW, DZ, DH, and BYL designed the study and interpreted the results. DW, DH, XS, YF performed molecular expression experiments. KXL, QXY, XYL contributed to collecting animal samples. DW and BYL conducted the patch-clamp experiments. DH, CPC, and HDL are responsible for correcting the proofs. DW, DZ, and BYL drafted and reviewed the manuscript. DW and BYL finalized the manuscript. BYL and DZ provided research funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, D., Zhao, D., Huang, D. et al. Estrogen-dependent depressor response of melatonin via baroreflex afferent function and intensification of PKC-mediated Nav1.9 activation. Acta Pharmacol Sin 43, 2313–2324 (2022). https://doi.org/10.1038/s41401-022-00867-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00867-w
Keywords
This article is cited by
-
Piezo1 channel activation facilitates baroreflex afferent neurotransmission with subsequent blood pressure reduction in control and hypertension rats
Acta Pharmacologica Sinica (2024)
-
New Perspectives on the Role and Therapeutic Potential of Melatonin in Cardiovascular Diseases
American Journal of Cardiovascular Drugs (2024)