Abstract
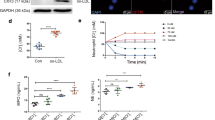
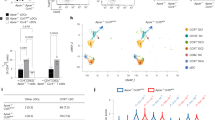
Platelet hyperactivity is essential for thrombus formation in coronary artery diseases (CAD). Dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) in patients with cystic fibrosis elevates intracellular Cl− levels ([Cl−]i) and enhanced platelet hyperactivity. In this study, we explored whether alteration of [Cl−]i has a pathological role in regulating platelet hyperactivity and arterial thrombosis formation. CFTR expression was significantly decreased, while [Cl−]i was increased in platelets from CAD patients. In a FeCl3-induced mouse mesenteric arteriole thrombosis model, platelet-specific Cftr-knockout and/or pre-administration of ion channel inhibitor CFTRinh-172 increased platelet [Cl−]i, which accelerated thrombus formation, enhanced platelet aggregation and ATP release, and increased P2Y12 and PAR4 expression in platelets. Conversely, Cftr-overexpressing platelets resulted in subnormal [Cl−]i, thereby decreasing thrombosis formation. Our results showed that clamping [Cl−]i at high levels or Cftr deficiency-induced [Cl−]i increasement dramatically augmented phosphorylation (Ser422) of serum and glucocorticoid-regulated kinase (SGK1), subsequently upregulated P2Y12 and PAR4 expression via NF-κB signaling. Constitutively active mutant S422D SGK1 markedly increased P2Y12 and PAR4 expression. The specific SGK1 inhibitor GSK-650394 decreased platelet aggregation in wildtype and platelet-specific Cftr knockout mice, and platelet SGK1 phosphorylation was observed in line with increased [Cl−]i and decreased CFTR expression in CAD patients. Co-transfection of S422D SGK1 and adenovirus-induced CFTR overexpression in MEG-01 cells restored platelet activation signaling cascade. Our results suggest that [Cl−]i is a novel positive regulator of platelet activation and arterial thrombus formation via the activation of a [Cl−]i-sensitive SGK1 signaling pathway. Therefore, [Cl−]i in platelets is a novel potential biomarker for platelet hyperactivity, and CFTR may be a potential therapeutic target for platelet activation in CAD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jackson SP. Arterial thrombosis—insidious, unpredictable and deadly. Nat Med. 2011;17:1423–36.
Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, et al. Platelets are versatile cells: new discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409–30.
Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the ‘magic bullet’. Nat Rev Drug Discov. 2003;2:775–89.
Xu XR, Carrim N, Neves MAD, McKeown T, Stratton TW, Coelho RMP, et al. Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb J. 2016;14:37–46.
Li BX, Dai X, Xu XR, Adili R, Neves MAD, Lei X, et al. In vitro assessment and phase I randomized clinical trial of anfibatide a snake venom derived anti-thrombotic agent targeting human platelet GPIbα. Sci Rep-Uk. 2021;11:1–17.
Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, et al. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707.
Guan Y, Wang G, Zhou J. The ClC-3 Cl− channel in cell volume regulation, proliferation and apoptosis in vascular smooth muscle cells. Trends Pharmacol Sci. 2006;27:290–6.
Testani JM, Hanberg JS, Arroyo JP, Brisco MA, ter Maaten JM, Wilson FP, et al. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18:660–8.
Naal T, Abuhalimeh B, Khirfan G, Dweik RA, Tang WHW, Tonelli AR. Serum chloride levels track with survival in patients with pulmonary arterial hypertension. Chest. 2018;154:541–9.
Harper MT, Poole AW. Chloride channels are necessary for full platelet phosphatidylserine exposure and procoagulant activity. Cell Death Dis. 2013;4:e969.
Vaitkevicius H, Turner I, Spalding A, Lockette W. Chloride increases adrenergic receptor-mediated platelet and vascular responses. Am J Hypertens. 2002;15:492–8.
O’Sullivan BP, Linden MD, Frelinger AL, Barnard MR, Spencer-Manzon M, Morris JE, et al. Platelet activation in cystic fibrosis. Blood. 2005;105:4635–41.
Mattoscio D, Evangelista V, De Cristofaro R, Recchiuti A, Pandolfi A, Di Silvestre S, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB J. 2010;24:3970–80.
Ortiz-Muñoz G, Yu MA, Lefrançais E, Mallavia B, Valet C, Tian JJ, et al. Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation. J Clin Invest. 2020;130:2041–53.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–55.
Zhang Y, Chen P, Guan W, Guo H, Qiu Z, Xu J, et al. Increased intracellular Cl− concentration promotes ongoing inflammation in airway epithelium. Mucosal Immunol. 2018;11:1149–57.
Zhang L, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol. 2006;95:2404–16.
Melis N, Tauc M, Cougnon M, Bendahhou S, Giuliano S, Rubera I, et al. Revisiting CFTR inhibition: a comparative study of CFTRinh‐172 and GlyH‐101 inhibitors. Br J Pharmacol. 2014;171:3716–27.
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, et al. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–8.
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, et al. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–58.
Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell (Camb). 2008;134:1019–29.
Li XY, Lv XF, Huang CC, Sun L, Ma MM, Liu C, et al. LRRC8A is essential for volume-regulated anion channel in smooth muscle cells contributing to cerebrovascular remodeling during hypertension. Cell Prolif. 2021:e13146.
Baba K, Shibata R, Sibuya M. Partial correlation and conditional correlation as measures of conditional independence. Aust Nz J Stat. 2004;46:657–64.
Budnik A, Henneberg M. Worldwide increase of obesity is related to the reduced opportunity for natural selection. PLoS One. 2017;12:e170098.
Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, et al. Evidence for direct CFTR inhibition by CFTRinh-172 based on Arg347 mutagenesis. Biochem J. 2008;413:135–42.
Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–34.
Chen B, Huang C, Lv X, Zheng H, Zhang Y, Sun L, et al. SGK1 mediates the hypotonic protective effect against H2O2-induced apoptosis of rat basilar artery smooth muscle cells by inhibiting the FOXO3a/Bim signaling pathway. Acta Pharmacol Sin. 2020;41:1073–84.
Ya-juan Z, Hua-qing Z, Bao-yi C, Lu S, Ming-ming MA, Guan-lei W, et al. WNK1 is required for proliferation induced by hypotonic challenge in rat vascular smooth muscle cells. Acta Pharmacol Sin. 2018;39:35–47.
Lang F, Gawaz M, Borst O. The serum- & glucocorticoid-inducible kinase in the regulation of platelet function. Acta Physiol. 2015;213:181–90.
Borst O, Schmidt E, Münzer P, Schönberger T, Towhid ST, Elvers M, et al. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood. 2012;119:251–61.
Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, et al. Platelets express activated P2Y12 receptor in patients with diabetes mellitus. Circulation. 2017;136:817–33.
Taylor KA, Wilson DGS, Harper MT, Pugh N. Extracellular chloride is required for efficient platelet aggregation. Platelets. 2018;29:79–83.
Walker B, Schmid E, Russo A, Schmidt EM, Burk O, Münzer P, et al. Impact of the serum- and glucocorticoid-inducible kinase 1 on platelet dense granule biogenesis and secretion. J Thromb Haemost. 2015;13:1325–34.
Bomberger JM, Coutermarsh BA, Barnaby RL, Sato JD, Chapline MC, Stanton BA, et al. Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval. PLoS One. 2014;9:e89599.
Cattaneo M. New P2Y12 inhibitors. Circulation. 2010;121:171–9.
Wang Y, Reheman A, Spring CM, Kalantari J, Marshall AH, Wolberg AS, et al. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124:4281–93.
Wang Y, Gallant RC, Ni H. Extracellular matrix proteins in the regulation of thrombus formation. Curr Opin Hematol. 2016;23:280–7.
Yang H, Reheman A, Chen P, Zhu G, Hynes RO, Freedman J, et al. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost. 2006;4:2230–7.
Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, et al. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3:875–83.
Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–19.
von Hundelshausen P, Weber C. Platelets as immune cells bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40.
Reverri EJ, Morrissey BM, Cross CE, Steinberg FM. Inflammation, oxidative stress, and cardiovascular disease risk factors in adults with cystic fibrosis. Free Radic Biol Med. 2014;76:261–77.
Yanda MK, Liu Q, Cebotaru L. A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J Biol Chem. 2018;293:11513–26.
Lei X, MacKeigan DT, Ni H. Control of data variations in intravital microscopy thrombosis models. J Thromb Haemost. 2020;18:2823–5.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82073848 and 81773722 to GLW, 82170231 to CZL, 81803522 to LH, 81903687 to LYZ, 62172452 to RMW, 82104160 to NP); The Science and Technology Program of Guangzhou City (No.201803010092 to GLW; China); Guangdong Natural Science Foundation (No. 2020A1515010045 to LYZ; China); Guangdong Provincial Department of Science and Technology (No.2017A020215104 to LYZ; China). The authors thank Dr. Zhong-ren Ding from the First Affiliated Hospital of Zhengzhou University, Cardiovascular Institute of Zhengzhou University for technical assistance. The authors thank Dr. Heyu Ni from St. Michael’s Hospital, University of Toronto for valuable suggestions and comments during the manuscript preparation.
Author information
Authors and Affiliations
Contributions
GLW, BZ, CZL, and HYY designed the study. HYY, CZ, LH, and CL performed the experiments and analyzed the data. NP, ML, HH, YZ, JL, LYZ, YSL, BZL, XQH, XFL, ZCL, JL, ZHL, and RMW assisted with the experiments. GLW, HYY, CZL, LH, and LW wrote the manuscript. YYG and BZ provided valuable suggestions. All authors have approved the final article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, Hy., Zhang, C., Hu, L. et al. Platelet CFTR inhibition enhances arterial thrombosis via increasing intracellular Cl− concentration and activation of SGK1 signaling pathway. Acta Pharmacol Sin 43, 2596–2608 (2022). https://doi.org/10.1038/s41401-022-00868-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00868-9