Abstract
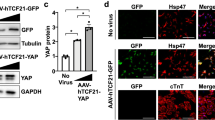
Aberrant activation of cardiac fibroblasts is the main cause and character of cardiac fibrosis, and inhibition of cardiac fibrosis becomes a promising treatment for cardiac diseases. Platelet-activating factor (PAF) and Hippo pathway is recently recognized as key signaling mechanisms in cardiovascular diseases. In this study we explored the potential roles of PAF and Hippo signaling pathway in cardiac fibrosis. Myocardial infarction (MI) was induced in mice by left anterior descending artery ligation. After 28 days, the mice were sacrificed, and the hearts were collected for analyses. We showed that PAF receptor (PAFR) and yes-associated protein 1 (YAP1, a key effector in the Hippo pathway) were significantly increased in the heart of MI mice. Increased expression of PAFR and YAP1 was also observed in angiotensin II (Ang II)-treated mouse cardiac fibroblasts. In mouse cardiac fibroblasts, forced expression of YAP1 increased cell viability, resulted in collagen deposition and promoted fibroblast-myofibroblast transition. We showed that PAF induced fibrogenesis through activation of YAP1 and promoted its nuclear translocation via interacting with PAFR, while YAP1 promoted the expression of PAFR by binding to and activating transcription factor TEAD1. More importantly, silencing PAFR or YAP1 by shRNA, or using transgenic mice to induce the conditional deletion of YAP1 in cardiac fibroblasts, impeded cardiac fibrosis and improved cardiac function in MI mice. Taken together, this study elucidates the role and mechanisms of PAFR/YAP1 positive feedback loop in cardiac fibrosis, suggesting a potential role of this pathway as novel therapeutic targets in cardiac fibrosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hinderer S, Schenke-Layland K. Cardiac fibrosis—a short review of causes and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:77–82.
Passaro F, Tocchetti CG, Spinetti G, Paudice F, Ambrosone L, Costagliola C, et al. Targeting fibrosis in the failing heart with nanoparticles. Adv Drug Deliv Rev. 2021;174:461–81.
Tao YK, Zhao SP, Yu PL, Shi J, Gu CD, Sun HT, et al. Elevated platelet activating factor level in ischemia-related arrhythmia and its electrophysiological effect on myocardium. Biomed Environ Sci. 2013;26:365–70.
Zheng GH, Xiong SQ, Mei LJ, Chen HY, Wang T, Chu JF. Elevated plasma platelet activating factor, platelet activating factor acetylhydrolase levels and risk of coronary heart disease or blood stasis syndrome of coronary heart disease in Chinese: a case control study: a case-control study. Inflammation. 2012;35:1419–28.
Penna C, Bassino E, Alloatti G. Platelet activating factor: the good and the bad in the ischemic/reperfused heart. Exp Biol Med. 2011;236:390–401.
Detopoulou P, Nomikos T, Fragopoulou E, Chrysohoou C, Antonopoulou S. Platelet activating factor in heart failure: potential role in disease progression and novel target for therapy. Curr Heart Fail Rep. 2013;10:122–9.
Detopoulou P, Nomikos T, Fragopoulou E, Antonopoulou S, Kotroyiannis I, Vassiliadou C, et al. Platelet activating factor (PAF) and activity of its biosynthetic and catabolic enzymes in blood and leukocytes of male patients with newly diagnosed heart failure. Clin Biochem. 2009;42:44–9.
Wang Y, Yu A, Yu FX. The Hippo pathway in tissue homeostasis and regeneration. Protein Cell. 2017;8:349–59.
Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–28.
Hong L, Li X, Zhou D, Geng J, Chen L. Role of Hippo signaling in regulating immunity. Cell Mol Immunol. 2018;15:1003–9.
Driskill JH, Pan D. The Hippo pathway in liver homeostasis and pathophysiology. Annu Rev Pathol. 2021;16:299–322.
Tamura T, Kodama T, Sato K, Murai K, Yoshioka T, Shigekawa M, et al. Dysregulation of PI3K and Hippo signaling pathways synergistically induces chronic pancreatitis via CTGF upregulation. J Clin Invest. 2021;131;e143414.
Li FL, Guan KL. The two sides of Hippo pathway in cancer. Semin Cancer Biol. 2021;S1044-579X(21)00200-5.
Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol. 2018;15:672–84.
Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110:13839–44.
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91.
Zhao X, He L, Li T, Lu Y, Miao Y, Liang S, et al. SRF expedites metastasis and modulates the epithelial to mesenchymal transition by regulating miR-199a-5p expression in human gastric cancer. Cell Death Differ. 2014;21:1900–13.
Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17.
Flinn MA, Link BA, O’Meara CC. Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration. Semin Cell Dev Biol. 2020;100:11–9.
Li Y, Feng J, Song S, Li H, Yang H, Zhou B, et al. gp130 controls cardiomyocyte proliferation and heart regeneration. Circulation. 2020;142:967–82.
Ma WY, Song RJ, Xu BB, Xu Y, Wang XX, Sun HY, et al. Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway. Acta Pharmacol Sin. 2021;42:921–31.
Yang Y, Nemoto EM, Harvey SA, Subbotin VM, Gandhi CR. Increased hepatic platelet activating factor (PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis. Gut. 2004;53:877–83.
Latchoumycandane C, Hanouneh M, Nagy LE, McIntyre TM. Inflammatory PAF receptor signaling initiates Hedgehog signaling and kidney fibrogenesis during ethanol consumption. PLoS ONE. 2015;10:e0145691.
Song M, Zhang H, Chen Z, Yang J, Li J, Shao S, et al. Shikonin reduces hepatic fibrosis by inducing apoptosis and inhibiting autophagy via the platelet-activating factor-mitogen-activated protein kinase axis. Exp Ther Med. 2021;21:28.
Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F, et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6:36019–31.
Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–63.
Aharonov A, Shakked A, Umansky KB, Savidor A, Genzelinakh A, Kain D, et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat Cell Biol. 2020;22:1346–56.
Xiao Y, Hill MC, Li L, Deshmukh V, Martin TJ, Wang J, et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev. 2019;33:1491–505.
Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, et al. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Invest. 2010;120:3555–67.
Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–88.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81900225, 82170299, 81870211, 81872863); the HMU Marshal Initiative Funding (HMUMIF-21023); the Major Scientific Fund Project of Heilongjiang Province (ZD2019H001); and the CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-078).
Author information
Authors and Affiliations
Contributions
HHL and HLS conceived the project, designed the experimental scheme and edited the manuscript. TYL, WS and LLL planned the experiments, composed the manuscript and managed experimental data. TYL, WS, XGZ, MQH, QZ, WHJ and XYS carried out cytological and molecular biological experiments, analyzed data and made charts. TYL, LLL, NY, JXG, XL, JZ were responsible for animals breeding, performed animal researches and executed data analysis. XGZ, JXG, JZ and NY assisted data analysis with constructive recommendation. YHZ and XLL gave suggestions on writing and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Li, Ty., Su, W., Li, Ll. et al. Critical role of PAFR/YAP1 positive feedback loop in cardiac fibrosis. Acta Pharmacol Sin 43, 2862–2872 (2022). https://doi.org/10.1038/s41401-022-00903-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00903-9
Keywords
This article is cited by
-
Single-cell RNA sequencing reveals the potential role of Postn(+) fibroblasts in promoting the progression of myocardial fibrosis after myocardial infarction
Scientific Reports (2025)
-
Mesenchymal stem cell-derived exosomal mir-21-5p inhibits YAP1 expression and improves outcomes in myocardial infarction
BMC Cardiovascular Disorders (2024)
-
YAP1 inhibits the senescence of alveolar epithelial cells by targeting Prdx3 to alleviate pulmonary fibrosis
Experimental & Molecular Medicine (2024)
-
m6A reader YTHDF1 promotes cardiac fibrosis by enhancing AXL translation
Frontiers of Medicine (2024)
-
Preventive effects of Ramelteon on bleomycin-induced pulmonary fibrosis in mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)