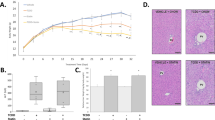
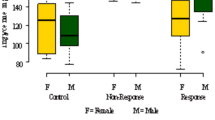
Abstract
Anterior gradient 2 (AGR2), a protein disulfide isomerase (PDI), is a multifunctional protein under physiological and pathological conditions. In this study we investigated the roles of AGR2 in regulating cholesterol biogenesis, lipid-lowering efficiency of lovastatin as well as in protection against hypercholesterolemia/statin-induced liver injury. We showed that AGR2 knockout significantly decreased hepatic and serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) in mice with whole-body or hepatocyte-specific Agr2-null mutant, compared with the levels in their wild-type littermates fed a normal chow diet (NCD) or high-fat diet (HFD). In contrast, mice with AGR2 overexpression (Agr2/Tg) exhibited an increased cholesterol level. Mechanistic studies revealed that AGR2 affected cholesterol biogenesis via activation of AKT/sterol regulatory element-binding protein-2 (SREBP2), to some extent, in a PDI motif-dependent manner. Moreover, elevated AGR2 led to a significant decrease in the lipid-lowering efficacy of lovastatin (10 mg· kg−1· d−1, ip, for 2 weeks) in mice with hypercholesterolemia (hyperCho), which was validated by results obtained from clinical samples in statin-treated patients. We showed that lovastatin had limited effect on AGR2 expression, but AGR2 was inducible in Agr2/Tg mice fed a HFD. Further investigations demonstrated that drug-induced liver toxicity and inflammatory reactions were alleviated in hypercholesterolemic Agr2/Tg mice, suggesting the dual functions of AGR2 in lipid management and hyperCho/statin-induced liver injury. Importantly, the AGR2-reduced lipid-lowering efficacy of lovastatin was attenuated, at least partially, by co-administration of a sulfhydryl-reactive compound allicin (20 mg· kg−1· d−1, ip, for 2 weeks). These results demonstrate a novel role of AGR2 in cholesterol metabolism, drug resistance and liver protection, suggesting AGR2 as a potential predictor for selection of lipid-lowering drugs in clinic.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Espinosa G, Lopez-Montero I, Monroy F, Langevin D. Shear rheology of lipid monolayers and insights on membrane fluidity. Proc Natl Acad Sci USA. 2011;108:6008–13.
Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–57.
Sozen E, Ozer NK. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol. 2017;12:456–61.
Chua NK, Coates HW, Brown AJ. Cholesterol, cancer, and rebooting a treatment for athlete’s foot. Sci Transl Med. 2018;10:eaat3741.
Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15:757–69.
Sabatine MS. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol. 2019;16:155–65.
Gitt AK, Drexel H, Feely J, Ferrieres J, Gonzalez-Juanatey JR, Thomsen KK, et al. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur J Prev Cardiol. 2012;19:221–30.
Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL 2nd, Goldstein LB, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–81.
Jones PH, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1:e001800.
Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, Michalak M. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–40.
Higa A, Mulot A, Delom F, Bouchecareilh M, Nguyen DT, Boismenu D, et al. Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J Biol Chem. 2011;286:44855–68.
Brychtova V, Mohtar A, Vojtesek B, Hupp TR. Mechanisms of anterior gradient-2 regulation and function in cancer. Semin Cancer Biol. 2015;33:16–24.
Fessart D, Domblides C, Avril T, Eriksson LA, Begueret H, Pineau R, et al. Secretion of protein disulphide isomerase AGR2 confers tumorigenic properties. Elife. 2016;5:e13887.
Jia M, Guo Y, Zhu D, Zhang N, Li L, Jiang J, et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-kappaB pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1622–33.
Wang Y, Jia M, Liang C, Sheng N, Wang X, Wang F, et al. Anterior gradient 2 increases long-chain fatty acid uptake via stabilizing FABP1 and facilitates lipid accumulation. Int J Biol Sci. 2021;17:834–47.
Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 2009;106:6950–5.
Wang D, Xu Q, Yuan Q, Jia M, Niu H, Liu X, et al. Proteasome inhibition boosts autophagic degradation of ubiquitinated-AGR2 and enhances the antitumor efficiency of bevacizumab. Oncogene. 2019;38:3458–74.
Eid W, Dauner K, Courtney KC, Gagnon A, Parks RJ, Sorisky A, et al. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc Natl Acad Sci USA. 2017;114:7999–8004.
Johnson C, Kastelic J, Thundathil J. Role of Akt and mammalian target of rapamycin signalling in insulin-like growth factor 1-mediated cell proliferation in porcine Sertoli cells. Reprod Fertil Dev. 2020;32:929–40.
Chaudhary HR, Brocks DR. The single dose poloxamer 407 model of hyperlipidemia; systemic effects on lipids assessed using pharmacokinetic methods, and its effects on adipokines. J Pharmacol Pharm Sci. 2013;16:65–73.
Zhang Q, Qian ZY, Zhou PH, Zhou XL, Zhang DL, He N, et al. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018;17:165.
Busch CJ, Hendrikx T, Weismann D, Jackel S, Walenbergh SM, Rendeiro AF, et al. Malondialdehyde epitopes are sterile mediators of hepatic inflammation in hypercholesterolemic mice. Hepatology. 2017;65:1181–95.
Meurer L, Cohen SM. Drug-induced liver injury from statins. Clin Liver Dis. 2020;24:107–19.
Bjornsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37:173–8.
Liu A, Wu Q, Guo J, Ares I, Rodriguez JL, Martinez-Larranaga MR, et al. Statins: adverse reactions, oxidative stress and metabolic interactions. Pharmacol Ther. 2019;195:54–84.
Maurel M, Obacz J, Avril T, Ding YP, Papadodima O, Treton X, et al. Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med. 2019;11:e10120.
Rana SV, Pal R, Vaiphei K, Sharma SK, Ola RP. Garlic in health and disease. Nutr Res Rev. 2011;24:60–71.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, et al. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics J. 2005;5:352–8.
Du Y, Wang S, Chen Z, Sun S, Zhao Z, Li X. Association of SLCO1B1 polymorphisms and atorvastatin safety and efficacy: a meta-analysis. Curr Pharm Des. 2018;24:4044–50.
Ten Kate GJ, Neefjes LA, Dedic A, Nieman K, Langendonk JG, Galema-Boers AJ, et al. The effect of LDLR-negative genotype on CT coronary atherosclerosis in asymptomatic statin treated patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2013;227:334–41.
Ahangari N, Ghayour Mobarhan M, Sahebkar A, Pasdar A. Molecular aspects of hypercholesterolemia treatment: current perspectives and hopes. Ann Med. 2018;50:303–11.
Tiwari V, Khokhar M. Mechanism of action of anti-hypercholesterolemia drugs and their resistance. Eur J Pharmacol. 2014;741:156–70.
Ma K, Malhotra P, Soni V, Hedroug O, Annaba F, Dudeja A, et al. Overactivation of intestinal SREBP2 in mice increases serum cholesterol. PLoS ONE. 2014;9:e84221.
Kim HJ, Lee J, Chung MY, Hong S, Park JH, Lee SH, et al. Piceatannol reduces resistance to statins in hypercholesterolemia by reducing PCSK9 expression through p300 acetyltransferase inhibition. Pharmacol Res. 2020;161:105205.
Tiemann K, Garri C, Lee SB, Malihi PD, Park M, Alvarez RM, et al. Loss of ER retention motif of AGR2 can impact mTORC signaling and promote cancer metastasis. Oncogene. 2019;38:3003–18.
Benincasa G, de Candia P, Costa D, Faenza M, Mansueto G, Ambrosio G, et al. Network medicine approach in prevention and personalized treatment of dyslipidemias. Lipids. 2021;56:259–68.
Su X, Cheng Y, Chang D. Lipid-lowering therapy: guidelines to precision medicine. Clin Chim Acta. 2021;514:66–73.
Crismaru I, Pantea Stoian A, Bratu OG, Gaman MA, Stanescu AMA, Bacalbasa N, et al. Low-density lipoprotein cholesterol lowering treatment: the current approach. Lipids Health Dis. 2020;19:85.
Han SN, Yang WH, Yin JJ, Tao HL, Zhang LR. Drug treatment of hyperlipidemia in Chinese patients: focus on the use of simvastatin and ezetimibe alone and in combination. Am J Cardiovasc Drugs. 2019;19:237–47.
Banach M, Patti AM, Giglio RV, Cicero AFG, Atanasov AG, Bajraktari G, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol. 2018;72:96–118.
Gupta A, Dong A, Lowe AW. AGR2 gene function requires a unique endoplasmic reticulum localization motif. J Biol Chem. 2012;287:4773–82.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81872896, 82173839 and 32000891), and Natural Science Foundation of Shandong Province (ZR2020QH363).
Author information
Authors and Affiliations
Contributions
NS and YQW performed the experiments, analyzed and interpreted the results, and wrote the paper. CFW collected clinical samples. MQJ and HMN analyzed and interpreted the results. QQL, YNW and XXZ assisted in animal experiments. DF was involved in data analysis. HQY designed the research, analyzed and interpreted the results, and wrote the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Sheng, N., Wang, Yq., Wang, Cf. et al. AGR2-induced cholesterol synthesis drives lovastatin resistance that is overcome by combination therapy with allicin. Acta Pharmacol Sin 43, 2905–2916 (2022). https://doi.org/10.1038/s41401-022-00909-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00909-3