Abstract
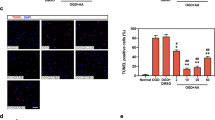
Metabolic cardiomyopathy (MC) is characterized by intracellular lipid accumulation and utilizing fatty acids as a foremost energy source, thereby leading to excess oxidative stress and mitochondrial dysfunction. There is no effective therapy available yet. In this study we investigated whether defective mitophagy contributed to MC and whether urolithin A (UA), a naturally occurring microflora-derived metabolite, could protect against MC in experimental obese mice. Mice were fed high fat diet for 20 weeks to establish a diet-induced obese model. We showed that mitochondrial autophagy or mitophagy was significantly downregulated in the heart of experimental obese mice. UA (50 mg·kg−1·d−1, for 4 weeks) markedly activated mitophagy and ameliorated MC in obese mice by gavage. In PA-challenged H9C2 cardiomyocytes, UA (5 μM) significantly increased autophagosomes and decreased autolysosomes. Furthermore, UA administration rescued PINK1/Parkin-dependent mitophagy and relieved mitochondrial defects in the heart of obese mice, which led to improving cardiac diastolic function and ameliorating cardiac remodelling. In PA-challenged primarily isolated cardiomyocytes, both application of mitophagy inhibitor Mdivi-1 (15 μM) and silencing of mitophagy gene Parkin blunted the myocardial protective effect of UA. In summary, our data suggest that restoration of mitophagy with UA ameliorates symptoms of MC, which highlights a therapeutic potential of UA in the treatment of MC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 Years. N Engl J Med. 2017;377:13–27.
Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126:1477–500.
Peterson LR, Gropler RJ. Metabolic and molecular imaging of the diabetic cardiomyopathy. Circ Res. 2020;126:1628–45.
Aminian A, Wilson R, Zajichek A, Tu C, Wolski KE, Schauer PR, et al. Cardiovascular outcomes in patients with type 2 diabetes and obesity: Comparison of gastric bypass, sleeve gastrectomy, and usual care. Diabetes Care. 2021;44:2552–63.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–38.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Ren J, Wu NN, Wang S, Sowers JR, Zhang Y. Obesity cardiomyopathy: Eevidence, mechanisms, and therapeutic implications. Physiol Rev. 2021;101:1745–807.
Ren J, Sun M, Zhou H, Ajoolabady A, Zhou Y, Tao J, et al. FUNDC1 interacts with FBXL2 to govern mitochondrial integrity and cardiac function through an IP3R3-dependent manner in obesity. Sci Adv. 2020;6:eabc8561. https://doi.org/10.1126/sciadv.abc8561.
Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124:121–41.
Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ Res. 2016;118:1736–51.
Shirihai OS, Song M, Dorn GW 2nd. How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116:1835–49.
Morales PE, Arias-Durán C, Ávalos-Guajardo Y, Aedo G, Verdejo HE, Parra V, et al. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Asp Med. 2020;71:100822.
Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 2015;116:1477–90.
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71.
Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Altern Med. 2013;2013:270418.
Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1:595–603.
Sorrentino V, Menzies KJ, Auwerx J. Repairing mitochondrial dysfunction in disease. Annu Rev Pharmacol Toxicol. 2018;58:353–89.
Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–8.
Zheng D, Liu Z, Zhou Y, Hou N, Yan W, Qin Y, et al. Urolithin B, a gut microbiota metabolite, protects against myocardial ischemia/reperfusion injury via p62/Keap1/Nrf2 signaling pathway. Pharmacol Res. 2020;153:104655.
Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–6.
Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Félix AA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–88.
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22:401–12.
Luan P, D’Amico D, Andreux PA, Laurila PP, Wohlwend M, Li H, et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci Transl Med. 2021;13:eabb0319. https://doi.org/10.1126/scitranslmed.abb0319.
Wu X, Qin Y, Zhu X, Liu D, Chen F, Xu S, et al. Increased expression of DRAM1 confers myocardial protection against ischemia via restoring autophagy flux. J Mol Cell Cardiol. 2018;124:70–82.
Peterson LR, Gropler RJ. Radionuclide imaging of myocardial metabolism. Circ Cardiovasc Imaging. 2010;3:211–22.
Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66.
Niemann B, Chen Y, Teschner M, Li L, Silber RE, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am Coll Cardiol. 2011;57:577–85.
Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 2019;124:1360–71.
Thomas A, Marek-Iannucci S, Tucker KC, Andres AM, Gottlieb RA. Decrease of cardiac parkin protein in obese mice. Front Cardiovasc Med. 2019;6:191.
Yu LM, Dong X, Xue XD, Xu S, Zhang X, Xu YL, et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J Pineal Res. 2021;70:e12698.
D’Amico D, Andreux PA, Valdés P, Singh A, Rinsch C, Auwerx J. Impact of the natural compound urolithin a on health, disease, and aging. Trends Mol Med. 2021;27:687–99.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China [81573429 to Dr. X Wu; U1601227 to Dr. Yu], and Natural Science Foundation of Guangdong Province [2021A1515012149 to Dr. X Wu].
Author information
Authors and Affiliations
Contributions
XQW and XYY conceived the research. XQW and JRH designed the experiments. JRH, MHZ, YJC, YLS, ZMG, ZJL, GPZ and YQ conducted experiments. XQW and JRH drafted the manuscript. XYY and XYD made critical revision of the manuscript for key intellectual content. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Material
Rights and permissions
About this article
Cite this article
Huang, Jr., Zhang, Mh., Chen, Yj. et al. Urolithin A ameliorates obesity-induced metabolic cardiomyopathy in mice via mitophagy activation. Acta Pharmacol Sin 44, 321–331 (2023). https://doi.org/10.1038/s41401-022-00919-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00919-1
Keywords
This article is cited by
-
Mitophagy in the pathogenesis and management of disease
Cell Research (2026)
-
Type 3 diabetes and metabolic reprogramming of brain neurons: causes and therapeutic strategies
Molecular Medicine (2025)
-
Mitochondrial quality control in cardiomyocytes: safeguarding the heart against disease and ageing
Nature Reviews Cardiology (2025)
-
Urolithin as a Metabolite of Ellagitannins and Ellagic Acid from Fruits and Nuts Produced by the Gut Microbiota: Its Role on Non-Communicable Diseases
Current Nutrition Reports (2025)
-
Decoding the gut microbiota-mitochondrial-inflammasome axis in cardiovascular disease
Molecular and Cellular Biochemistry (2025)