Abstract
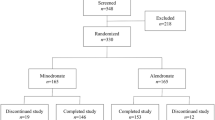
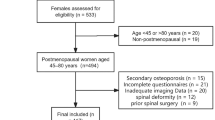
The current study evaluated the efficacy and safety of a denosumab biosimilar, QL1206 (60 mg), compared to placebo in postmenopausal Chinese women with osteoporosis and high fracture risk. At 31 study centers in China, a total of 455 postmenopausal women with osteoporosis and high fracture risk were randomly assigned to receive QL1206 (60 mg subcutaneously every 6 months) or placebo. From baseline to the 12-month follow-up, the participants who received QL1206 showed significantly increased bone mineral density (BMD) values (mean difference and 95% CI) in the lumbar spine: 4.780% (3.880%, 5.681%), total hip :3.930% (3.136%, 4.725%), femoral neck 2.733% (1.877%, 3.589%) and trochanter: 4.058% (2.791%, 5.325%) compared with the participants who received the placebo. In addition, QL1206 injection significantly decreased the serum levels of C-terminal crosslinked telopeptides of type 1 collagen (CTX): −77.352% (−87.080%, −66.844%), and N-terminal procollagen of type l collagen (P1NP): −50.867% (−57.184%, −45.217%) compared with the placebo over the period from baseline to 12 months. No new or unexpected adverse events were observed. We concluded that compared with placebo, QL1206 effectively increased the BMD of the lumbar spine, total hip, femoral neck and trochanter in postmenopausal Chinese women with osteoporosis and rapidly decreased bone turnover markers. This study demonstrated that QL1206 has beneficial effects on postmenopausal Chinese women with osteoporosis and high fracture risk.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–76.
Wang Y, Tao Y, Hyman ME, Li J, Chen Y. Osteoporosis in China. Osteoporos Int. 2009;20:1651–62.
Gao C, Xu Y, Li L, Gu WQ, Yi CT, Zhu Q, et al. Prevalence of osteoporotic vertebral fracture among community-dwelling elderly in Shanghai. Chin Med J. 2019;132:1749–51.
Xia WB, He SL, Xu L, Liu AM, Jiang Y, Li M, et al. Rapidly increasing rates of hip fracture in Beijing, China. J Bone Min Res. 2012;27:125–9.
Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5.
McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31.
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65.
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–23.
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–10.
Koh JM, Chung DJ, Chung YS, Kang MI, Kim IJ, Min YK, et al. Assessment of denosumab in Korean postmenopausal women with osteoporosis: randomized, double-blind, placebo-controlled trial with open-label extension. Yonsei Med J. 2016;57:905–14.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Ferrari S, Adachi JD, Lippuner K, Zapalowski C, Miller PD, Reginster JY, et al. Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos Int. 2015;26:2763–71.
Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab. 2014;99:2599–607.
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26:1–46.
Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44.
Zhang H, Wu M, Zhu X, Li C, Li X, Sun J, et al. A phase I, randomized, single-dose study to evaluate the biosimilarity of QL1206 to denosumab among Chinese healthy subjects. Front Pharmacol. 2020;11:01329.
Nakamura T, Matsumoto T, Sugimoto T, Shiraki M. Dose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos Int. 2012;23:1131–40.
Pitale S, Thomas M, Rathi G, Deshmukh V, Kumar P, Reddy S, et al. A randomized placebo-controlled trial of the efficacy of denosumab in Indian postmenopausal women with osteoporosis. Indian J Endocrinol Metab. 2015;19:148–54.
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–9.
Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Min Res. 2011;26:530–7.
Acknowledgements
We thank the study participants for their invaluable cooperation. The study was sponsored by Qilu Pharmaceutical Co. Ltd, Jinan, China.
Author information
Authors and Affiliations
Contributions
ZLZ, AJC, QC, DHT, JMY, BWW, YNH, LM, QZ, HY, SGY, KQZ, XLZ, HL, YP, ZY, RCD, LH, LC, YFS, ZLD, LY, BB, MZ, YLC, YKZ, ZJL, ZZ, CQY, YBL, GW, and CCH conceived and designed research; HZ, JMG, ZLZ, AJC, QC, DHT, JMY, BWW, YNH, LM, QZ, HY, SGY, KQZ, XLZ, HL, YP, ZY, RCD, LH, LC, YFS, ZLD, LY, BB, MZ, YLC, YKZ, ZJL, ZZ, CQY, YBL, and GW collected data and conducted research; HZ, JMG, ZLZ, AJC, QC, DHT, JMY, BWW, YNH, LM, QZ, HY, SGY, KQZ, XLZ, HL, YP, ZY, RCD, LH, LC, YFS, ZLD, LY, XXL, and ZJW analysed and interpreted data; HZ, JMG, ZLZ, AJC, and QC wrote the initial paper; HZ, JMG, ZLZ, AJC, QC, DHT, JMY, BWW, YNH, LM, QZ, HY, SGY, KQZ, XLZ, HL, YP, ZY, RCD, LH, LC, YFS, ZLD, LY, BB, MZ, YLC, YKZ, ZJL, ZZ, CQY, YBL, and GW revised the paper; ZLZ was primarily responsible for the final content. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
CCH, ZJW, and XXL are employees of the study sponsor (Qilu Pharmaceutical Co., Ltd) at the time of study. The other authors declare that they have no competing interests.
Ethics approval
This study was approved by the independent ethics committees of 31 participating sites.
Rights and permissions
About this article
Cite this article
Zhang, H., Gu, Jm., Chao, Aj. et al. A phase III randomized, double-blind, placebo-controlled trial of the denosumab biosimilar QL1206 in postmenopausal Chinese women with osteoporosis and high fracture risk. Acta Pharmacol Sin 44, 446–453 (2023). https://doi.org/10.1038/s41401-022-00954-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00954-y
Keywords
This article is cited by
-
The clinical trial landscape of anti-RANKL agents for osteoporosis: current status and future directions
Osteoporosis International (2025)
-
Efficacy and safety of denosumab biosimilar CMAB807 versus Prolia® in Chinese postmenopausal women with osteoporosis
Endocrine (2025)
-
A phase III clinical trial of monthly minodronate in the treatment of Chinese postmenopausal women with osteoporosis
Acta Pharmacologica Sinica (2025)
-
Efficacy and safety of candidate biosimilar CT-P41 versus reference denosumab: a double-blind, randomized, active-controlled, Phase 3 trial in postmenopausal women with osteoporosis
Osteoporosis International (2024)