Abstract
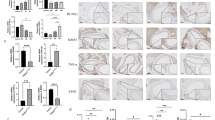
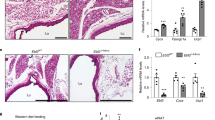
Abdominal aortic aneurysm (AAA) is a dangerous vascular disease without any effective drug therapies so far. Emerging evidence suggests the phenotypic differences in perivascular adipose tissue (PVAT) between regions of the aorta are implicated in the development of atherosclerosis evidenced by the abdominal aorta more vulnerable to atherosclerosis than the thoracic aorta in large animals and humans. The prevalence of thoracic aortic aneurysms (TAA) is much less than that of abdominal aortic aneurysms (AAA). In this study we investigated the effect of thoracic PVAT (T-PVAT) transplantation on aortic aneurysm formation and the impact of T-PVAT on vascular smooth muscle cells. Calcium phosphate-induced mouse AAA model was established. T-PVAT (20 mg) was implanted around the abdominal aorta of recipient mice after removal of endogenous abdominal PVAT (A-PVAT) and calcium phosphate treatment. Mice were sacrificed two weeks after the surgery and the maximum external diameter of infrarenal aorta was measured. We found that T-PVAT displayed a more BAT-like phenotype than A-PVAT; transplantation of T-PVAT significantly attenuated calcium phosphate-induced abdominal aortic dilation and elastic degradation as compared to sham control or A-PVAT transplantation. In addition, T-PVAT transplantation largely preserved smooth muscle cell content in the abdominal aortic wall. Co-culture of T-PVAT with vascular smooth muscle cells (VSMCs) significantly inhibited H2O2− or TNFα plus cycloheximide-induced VSMC apoptosis. RNA sequencing analysis showed that T-PVAT was enriched by browning adipocytes and anti-apoptotic secretory proteins. We further verified that the secretome of mature adipocytes isolated from T-PVAT significantly inhibited H2O2− or TNFα plus cycloheximide-induced VSMC apoptosis. Using proteomic and bioinformatic analyses we identified cartilage oligomeric matrix protein (COMP) as a secreted protein significantly increased in T-PVAT. Recombinant COMP protein significantly inhibited VSMC apoptosis. We conclude that T-PVAT exerts anti-apoptosis effect on VSMCs and attenuates AAA formation, which is possibly attributed to the secretome of browning adipocytes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Howard DP, Banerjee A, Fairhead JF, Handa A, Silver LE, Rothwell PM, et al. Population-based study of incidence of acute abdominal aortic aneurysms with projected impact of screening strategy. J Am Heart Assoc. 2015;4:e001926.
Chang L, Garcia-Barrio MT, Chen YE. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2020;40:1094–109.
Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–96.
Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–6.
Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–71.
Manka D, Chatterjee TK, Stoll LL, Basford JE, Konaniah ES, Srinivasan R, et al. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arterioscler Thromb Vasc Biol. 2014;34:1723–30.
Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheit-Nestler M, Czepluch FS, et al. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol. 2013;33:980–7.
Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–78.
Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225:99–104.
Meekel JP, Dias-Neto M, Bogunovic N, Conceicao G, Sousa-Mendes C, Stoll GR, et al. Inflammatory gene expression of human perivascular adipose tissue in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2021;61:1008–16.
Liu CL, Ren J, Wang Y, Zhang X, Sukhova GK, Liao M, et al. Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice. Eur Heart J. 2019;41:2456–68.
Zhang ZB, Ruan CC, Lin JR, Xu L, Chen XH, Du YN, et al. Perivascular adipose tissue-derived PDGF-D contributes to aortic aneurysm formation during obesity. Diabetes. 2018;67:1549–60.
Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–52.
Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–28.
Mathur A, Mohan V, Ameta D, Gaurav B, Haranahalli P. Aortic aneurysm. J Transl Int Med. 2016;4:35–41.
Yamanouchi D, Morgan S, Stair C, Seedial S, Lengfeld J, Kent KC, et al. Accelerated aneurysmal dilation associated with apoptosis and inflammation in a newly developed calcium phosphate rodent abdominal aortic aneurysm model. J Vasc Surg. 2012;56:455–61.
Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–11.
Raffort J, Lareyre F, Clement M, Hassen-Khodja R, Chinetti G, Mallat Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017;14:457–71.
Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–32.
Quintana RA, Taylor WR. Cellular mechanisms of aortic aneurysm formation. Circ Res. 2019;124:607–18.
Li X, Ballantyne LL, Yu Y, Funk CD. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J: Off Publ Federation Am Societies Exp Biol. 2019;33:12704–22.
Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci. 2012;122:1–12.
Gagarina V, Carlberg AL, Pereira-Mouries L, Hall DJ. Cartilage oligomeric matrix protein protects cells against death by elevating members of the IAP family of survival proteins. J Biol Chem. 2008;283:648–59.
Dias-Neto M, Meekel JP, van Schaik TG, Hoozemans J, Sousa-Nunes F, Henriques-Coelho T, et al. High density of periaortic adipose tissue in abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2018;56:663–71.
Thanigaimani S, Golledge J. Role of adipokines and perivascular adipose tissue in abdominal aortic aneurysm: a systematic review and meta-analysis of animal and human observational studies. Front Endocrinol (Lausanne). 2021;12:618434.
Yoshida S, Fuster JJ, Walsh K. Adiponectin attenuates abdominal aortic aneurysm formation in hyperlipidemic mice. Atherosclerosis. 2014;235:339–46.
Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 2019;30:963–75.e7.
Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–37.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82170492, 81900387), Guangdong Basic and Applied Basic Research Found (2019A1515011806), Science and Technology Program of Guangzhou City of China (202102010302). The mass spectrometry analysis was performed by the Bioinformatics and Omics Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University.
Author information
Authors and Affiliations
Contributions
ZYL, YXC and JFW directed the project. ZYL designed the experiments and drafted the manuscript. CLH, YNH, and LY, JPL, ZHZ, ZQH, SXC performed the experiments and analyzed the data. YLZ helped with data curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Cl., Huang, Yn., Yao, L. et al. Thoracic perivascular adipose tissue inhibits VSMC apoptosis and aortic aneurysm formation in mice via the secretome of browning adipocytes. Acta Pharmacol Sin 44, 345–355 (2023). https://doi.org/10.1038/s41401-022-00959-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00959-7
Keywords
This article is cited by
-
Perivascular adipose tissue dysfunction contributes to thoracic aortic aneurysm development
Cardiovascular Diabetology (2025)
-
Perivascular adipose tissue: a central player in the triad of diabetes, obesity, and cardiovascular health
Cardiovascular Diabetology (2024)