Abstract
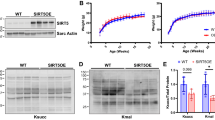
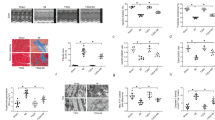
Sirtuin3 (SIRT3), a class III histone deacetylase, is implicated in various cardiovascular diseases as a novel therapeutic target. SIRT3 has been proven to be cardioprotective in a model of Ang II-induced cardiac hypertrophy. However, a few small-molecule compounds targeting deacetylases could activate SIRT3. In this study, we generated a novel SIRT3 activator, 3-(2-bromo-4-hydroxyphenyl)-7-hydroxy-2H-chromen-2-one (SZC-6), through structural optimization of the first SIRT3 agonist C12. We demonstrated that SZC-6 directly bound to SIRT3 with Kd value of 15 μM, and increased SIRT3 deacetylation activity with EC50 value of 23.2 ± 3.3 µM. In neonatal rat cardiomyocytes (NRCMs), pretreatment with SZC-6 (10, 20, 40 µM) dose-dependently attenuated isoproterenol (ISO)-induced hypertrophic responses. Administration of SZC-6 (20, 40 and 60 mg·kg−1·d−1, s.c.) for 2 weeks starting from one week prior ISO treatment dose-dependently reversed ISO-induced impairment of diastolic and systolic cardiac function in wild-type mice, but not in SIRT3 knockdown mice. We showed that SZC-6 (10, 20, 40 µM) dose-dependently inhibited cardiac fibroblast proliferation and differentiation into myofibroblasts, which was abolished in SIRT3-knockdown mice. We further revealed that activation of SIRT3 by SZC-6 increased ATP production and rate of mitochondrial oxygen consumption, and reduced ROS, improving mitochondrial function in ISO-treated NRCMs. We also found that SZC-6 dose-dependently enhanced LKB1 phosphorylation, thereby promoting AMPK activation to inhibit Drp1-dependent mitochondrial fragmentation. Taken together, these results demonstrate that SZC-6 is a novel SIRT3 agonist with potential value in the treatment of cardiac hypertrophy partly through activation of the LKB1-AMPK pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lu JQ, Zhang H, Chen X, Zou Y, Li JS, Wang L, et al. A small molecule activator of SIRT3 promotes deacetylation and activation of manganese superoxide dismutase. Free Radic Biol Med. 2017;112:287–97.
Lin ZQ, Murtaza I, Wang K, Jiao JQ, Gao J, Li PF. MiR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:12103–8.
Ding YQ, Zhang YH, Lu J, Li B, Yu WJ, Yue ZB, et al. MicroRNA-214 contributes to Ang II-induced cardiac hypertrophy by targeting SIRT3 to provoke mitochondrial malfunction. Acta Pharmacol Sin. 2021;42:1422–36.
Kim TS, Jin YB, Kim YS, Kim S, Kim JK, Lee HM, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15:1356–75.
Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139–49.
Gu DC, Chen CY, Zhao ML, Zhao LM, Duan XW, Duan J, et al. Identification of HDA15-PIF1 as a key repression module directing the transcriptional network of seed germination in the dark. Nucleic Acids Res. 2017;45:7137–50.
Jiang WQ, Wang SW, Xiao MT, Lin Y, Zhou LS, Lei QY, et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43:33–44.
Wellman AS, Metukuri MR, Kazgan N, Xu XJ, Xu Q, Ren NSX, et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology. 2017;153:772–86.
Jeong SM, Xiao CY, Finley LWS, Lahusen T, Souza AL, Pierce K, et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell. 2013;23:450–63.
Rardin MJ, He WJ, Nishida Y, Newman JC, Carrico C, Danielson SR, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–33.
Zhang XK, Ji RP, Liao XH, Castillero E, Kennel PJ, Brunjes DL, et al. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation. 2018;137:2052–67.
Zhang J, Xiang H, Liu J, Chen Y, He RR, Liu B. Mitochondrial sirtuin 3: new emerging biological function and therapeutic target. Theranostics. 2020;10:8315–42.
Wei T, Gao J, Huang C, Song B, Sun M, Shen WL. SIRT3 (sirtuin-3) prevents Ang II (angiotensin II)-induced macrophage metabolic switch improving perivascular adipose tissue function. Arterioscler Thromb Vasc Biol. 2021;41:714–30.
Sun W, Liu CX, Chen QH, Liu N, Yan YY, Liu B. SIRT3: a new rregulator of cardiovascular diseases. Oxid Med Cell Longev. 2018;2018:1–11.
Liu LJ, Nam M, Fan W, Akie TE, Hoaglin DC, Gao GP, et al. Nutrient sensing by the mitochondrial transcription machinery dictates oxidative phosphorylation. J Clin Invest. 2014;124:768–84.
Zhang X, Ren X, Zhang Q, Li Z, Ma S, Bao J, et al. PGC-1α/ERRα-Sirt3 pathway regulates DAergic neuronal death by directly deacetylating SOD2 and ATP Synthase β. Antioxid Redox Signal. 2016;24:312–28.
Vassilopoulos A, Pennington J, Andresson T, Rees D, Bosley A, Fearnley I, et al. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal. 2014;21:551–64.
Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–90.
Jing EX, Emanuelli B, Boucher J, Carmona J, Kim B, Kahn CR. Sirt3, a novel nutrient sensor and regulator of insulin signaling in diabetes and insulin resistance. Diabetes. 2008;57:A44–A.
Tomczyk M, Cheung K, Xiang B, Tamanna N, Fonseca Teixeira A, Agarwal P, et al. Mitochondrial sirtuin-3 (SIRT3) prevents doxorubicin-induced dilated cardiomyopathy by modulating protein acetylation and oxidative stress. J Biol Chem. 2022;15:e008547.
Morishima C, Shuhart MC, Wang CC, Paschal DM, Apodaca MC, Liu YZ, et al. Silymarin inhibits in vitro T-cell proliferation and cytokine production in hepatitis c virus infection. Gastroenterology. 2010;138:671–U332.
Li Y, Ye ZC, Lai WY, Rao JL, Huang WB, Zhang XH, et al. Activation of sirtuin 3 by silybin attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Front Pharmacol. 2017;8:178.
Pillai VB, Kanwal A, Fang YH, Sharp WW, Samant S, Arbiser J, et al. Honokiol, an activator of Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin-induced cardiomyopathy in mice. Oncotarget. 2017;8:34082–98.
Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656.
Wang M, Li Y, Ni CW, Song GJ. Honokiol attenuates oligomeric amyloid beta(1-42)-induced alzheimer’s disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor kappa-B signaling pathway. Cell Physiol Biochem. 2017;43:69–81.
Li J, Huang J, Lu J, Guo Z, Li Z, Gao H, et al. Sirtuin 1 represses PKC-ζ activity through regulating interplay of acetylation and phosphorylation in cardiac hypertrophy. Br J Pharmacol. 2019;176:416–35.
Wang PX, Wang LP, Lu J, Hu YH, Wang QQ, Li ZZ, et al. SESN2 protects against doxorubicin-induced cardiomyopathy via rescuing mitophagy and improving mitochondrial function. J Mol Cell Cardiol. 2019;133:125–37.
Fu J, Gao J, Pi R, Liu P. An optimized protocol for culture of cardiomyocyte from neonatal rat. Cytotechnology. 2005;49:109–16.
Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562:423–8.
Li X, Dai F, Wang H, Wei G, Jiang Q, Yin P, et al. PCSK9 participates in oxidized-low density lipoprotein-induced myocardial injury through mitochondrial oxidative stress and Drp1-mediated mitochondrial fission. Clin Transl Med. 2022;12:e729.
Huang ZM, Zhao JX, Deng W, Chen YY, Shang JL, Song K, et al. Identification of a cellularly active SIRT6 allosteric activator. Nat Chem Biol. 2018;14:1118–26.
Yoshino J, Mills KF, Yoon MJ, Imai SI. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-Induced diabetes in mice. Cell Metab. 2011;14:528–36.
Cheng AW, Yang Y, Zhou Y, Maharana C, Lu DY, Peng W, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23:128–42.
Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–U193.
Ma LL, Kong FJ, Ma YJ, Guo JJ, Wang SJ, Dong Z, et al. Hypertrophic preconditioning attenuates post-myocardial infarction injury through deacetylation of isocitrate dehydrogenase 2. Acta Pharmacol Sin. 2021;42:2004–15.
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–71.
Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, et al. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2007;100:766–8.
Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–27.
Hu TP, Xu FP, Li YJ, Luo JD. Simvastatin inhibits leptin-induced hypertrophy in cultured neonatal rat cardiomyocytes. Acta Pharmacol Sin. 2006;27:419–22.
Xu HX, Cui SM, Zhang YM, Ren J. Mitochondrial Ca2+ regulation in the etiology of heart failure: physiological and pathophysiological implications. Acta Pharmacol Sin. 2020;41:1301–9.
Jo DS, Park SJ, Kim AK, Park NY, Kim JB, Bae JE, et al. Loss of HSPA9 induces peroxisomal degradation by increasing pexophagy. Autophagy. 2020;16:1989–2003.
Jin JY, Wei XX, Zhi XL, Wang XH, Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. 2021;42:655–64.
Wang LT, He PC, Li AQ, Cao KX, Yan JW, Guo S, et al. Caffeine promotes angiogenesis through modulating endothelial mitochondrial dynamics. Acta Pharmacol Sin. 2021;42:2033–45.
Qi DK, Atsina K, Qu LT, Hu XY, Wu XH, Xu B, et al. The vestigial enzyme D-dopachrome tautomerase protects the heart against ischemic injury. J Clin Invest. 2014;124:3540–50.
Zhang T, Liu JX, Shen SN, Tong Q, Ma XJ, Lin LG. SIRT3 promotes lipophagy and chaperon-mediated autophagy to protect hepatocytes against lipotoxicity. Cell Death Differ. 2020;27:329–44.
Seok HY, Chen JH, Kataoka M, Huang ZP, Ding J, Yan JL, et al. Loss of MicroRNA-155 Protects the heart from pathological cardiac hypertrophy. Circ Res. 2014;114:1585–95.
Jensen MK, Bartz TM, Mukamal KJ, Djousse L, Kizer JR, Tracy RP, et al. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older aadults the cardiovascular health study. Diabetes Care. 2013;36:1222–8.
Nguyen TTM, Wong R, Menazza S, Sun JH, Chen Y, Wang GH, et al. Cyclophilin d modulates mitochondrial acetylome. Circ Res. 2013;113:1308–19.
Yue ZB, Ma YZ, You J, Li ZM, Ding YQ, He P, et al. NMNAT3 is involved in the protective effect of SIRT3 in Ang II-induced cardiac hypertrophy. Exp Cell Res. 2016;347:261–73.
Li ZZ, Zhang XY, Guo Z, Zhong Y, Wang PX, Li JY, et al. SIRT6 suppresses NFATc4 expression and activation in cardiomyocyte hypertrophy. Front Pharmacol. 2019;9:1519.
Shen PY, Feng XJ, Zhang XY, Huang XY, Liu SL, Lu X, et al. SIRT6 suppresses phenylephrine-induced cardiomyocyte hypertrophy though inhibiting p300. J Pharm Sci. 2016;132:31–40.
Tang XQ, Chen XF, Wang NY, Wang XM, Liang ST, Zheng W, et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation. 2017;136:2051–67.
Zhang MM, Zhao ZJ, Shen M, Zhang YM, Duan JH, Guo YJ, et al. Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1962–72.
Srivastava SP, Li JP, Kitada M, Fujita H, Yamada Y, Goodwin JE, et al. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis. 2018;9:997.
Lu J, Zhang H, Chen X, Zou Y, Li J, Wang L, et al. A small molecule activator of SIRT3 promotes deacetylation and activation of manganese superoxide dismutase. Free Radic Biol Med. 2017;112:287–97.
Akter R, Afrose A, Rahman MR, Chowdhury R, Nirzhor SSR, Khan RI, et al. A comprehensive analysis into the therapeutic application of natural products as SIRT6 modulators in alzheimer’s disease, aging, ccancer, inflammation, and diabetes. Int J Mol Sci. 2021;22:4180.
Heger J, Hirschhäuser C, Bornbaum J, Sydykov A, Dempfle A, Schneider A, et al. Cardiomyocytes-specific deletion of monoamine oxidase B reduces irreversible myocardial ischemia/reperfusion injury. Free Radic Biol Med. 2021;165:14–23.
Zhang F, Klebansky B, Fine R, Xu H, Pronin A, Liu H, et al. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA. 2008;105:20930–4.
Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–8.
Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure implications beyond ATP production. Circ Res. 2013;113:709–24.
Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Suthammarak W, Gong GH, et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart Failure. Cell Metab. 2013;18:239–50.
Zhu X, Shen W, Yao K, Wang H, Liu B, Li T, et al. Fine-tuning of PGC1α expression regulates cardiac function and longevity. Circ Res. 2019;125:707–19.
Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X, et al. Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation. 2017;136:2248–66.
Zhou H, Hu N, Cao F, Chen YD, Ren J. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission/VDAC1/HK2/mPTP/Mitophagy axis. J Pineal Res. 2017;63:e12413.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U21A20419, 82173808, 82150204, 81872860, 82104157), Natural Science Foundation of Guangdong Province (2021B1515020100, 2022A1515012322, 2016A030311033), the Basic and Applied Basic Research Foundation of Guangdong Province (2020A1515410003), National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province, 2017B090903004), Guangzhou Basic and Applied Basic Research Project (202102020173) and Guangdong Provincial Key Laboratory of Construction Foundation (2017B030314030), the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (22qntd4510).
Author information
Authors and Affiliations
Contributions
PQL, YZ and XLZ were responsible for the experimental design and supervision of this project. ZYL performed most of the experiments and was the main writer of the manuscript. ZYL and PXW contributed to animal experiments. GQL contributed to the synthesis of compounds. JL contributed to the data analysis and manuscript writing. All authors made important suggestions regarding the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Zy., Lu, Gq., Lu, J. et al. SZC-6, a small-molecule activator of SIRT3, attenuates cardiac hypertrophy in mice. Acta Pharmacol Sin 44, 546–560 (2023). https://doi.org/10.1038/s41401-022-00966-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00966-8
Keywords
This article is cited by
-
ML335 inhibits TWIK2 channel-mediated potassium efflux and attenuates mitochondrial damage in MSU crystal-induced inflammation
Journal of Translational Medicine (2024)