Abstract
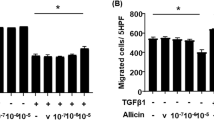
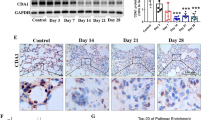
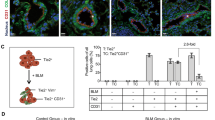
Pulmonary fibrosis (PF) is a chronic interstitial lung disease with no effective therapies. Galectin-3 (Gal-3), a marker of oxidative stress, plays a key role in the pathogenesis of PF. Fibroblast-myofibroblast differentiation (FMD) is an important source of fibrotic cells in PF. Previous studies showed that melatonin (MT) exerted anti-fibrotic effect in many diseases including PF through its antioxidant activity. In the present study we investigated the relationships among Gal-3, NRF2, ROS in FMD and their regulation by MT. We established an in vitro model of FMD in TGF-β1-treated human fetal lung fibroblast1 (HFL1) cells and a PF mouse model via bleomycin (BLM) intratracheal instillation. We found that Gal-3 expression was significantly increased both in vitro and in vivo. Knockdown of Gal-3 in HFL1 cells markedly attenuated TGF-β1-induced FMD process and ROS accumulation. In TGF-β1-treated HFL1 cells, pretreatment with NRF2-specific inhibitor ML385 (5 μM) significantly increased the levels of Gal-3, α-SMA and ROS, suggesting that the expression of Gal-3 was regulated by NRF2. Treatment with NRF2-activator MT (250 μM) blocked α-SMA and ROS accumulation accompanied by reduced Gal-3 expression. In BLM-induced PF model, administration of MT (5 mg·kg−1·d−1, ip for 14 or 28 days) significantly attenuated the progression of lung fibrosis through up-regulating NRF2 and down-regulating Gal-3 expression in lung tissues. These results suggest that Gal-3 regulates TGF-β1-induced pro-fibrogenic responses and ROS production in FMD, and MT activates NRF2 to block FMD process by down-regulating Gal-3 expression. This study provides a useful clue for a clinical strategy to prevent PF.

Graphic abstract of the mechanisms. MT attenuated BLM-induced PF via activating NRF2 and inhibiting Gal-3 expression.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–62.
Guler SA, Lindell KO, Swigris J, Ryerson CJ. What is idiopathic pulmonary fibrosis? IPF Part 1. Am J Respir Crit Care Med. 2021;203:P5–P6.
Le Pavec J, Dauriat G, Gazengel P, Dolidon S, Hanna A, Feuillet S, et al. Lung transplantation for idiopathic pulmonary fibrosis. Presse Med. 2020;49:104026.
Lechowicz K, Drozdzal S, Machaj F, Rosik J, Szostak B, Zegan-Baranska M, et al. COVID-19: The potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. 2020;9:1917–36.
George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–15.
John AE, Joseph C, Jenkins G, Tatler AL. COVID-19 and pulmonary fibrosis: a potential role for lung epithelial cells and fibroblasts. Immunol Rev. 2021;302:228–40.
Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185:537–46.
Hirani N, MacKinnon AC, Nicol L, Ford P, Schambye H, Pedersen A, et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2021;57:2002559.
Lubrano V, Balzan S. Role of oxidative stress-related biomarkers in heart failure: galectin 3, alpha1-antitrypsin and LOX-1: new therapeutic perspective? Mol Cell Biochem. 2020;464:143–52.
Fulton DJR, Li X, Bordan Z, Wang Y, Mahboubi K, Rudic RD, et al. Galectin-3: a harbinger of reactive oxygen species, fibrosis, and inflammation in pulmonary arterial hypertension. Antioxid Redox Signal. 2019;31:1053–69.
Mendonca HR, Carpi-Santos R, da Costa Calaza K, Blanco Martinez AM. Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res. 2020;15:625–35.
Alves CM, Silva DA, Azzolini AE, Marzocchi-Machado CM, Lucisano-Valim YM, Roque-Barreira MC, et al. Galectin-3 is essential for reactive oxygen species production by peritoneal neutrophils from mice infected with a virulent strain of Toxoplasma gondii. Parasitology. 2013;140:210–9.
Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995;154:3479–87.
Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–28.
Suzuki Y, Inoue T, Yoshimaru T, Ra C. Galectin-3 but not galectin-1 induces mast cell death by oxidative stress and mitochondrial permeability transition. Biochim Biophys Acta. 2008;1783:924–34.
Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26.
Zhang Z, Qu J, Zheng C, Zhang P, Zhou W, Cui W, et al. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 2018;9:83.
Qu J, Zhang Z, Zhang P, Zheng C, Zhou W, Cui W, et al. Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF. J Cell Physiol. 2019;234:8862–72.
Li N, Wang Z, Gao F, Lei Y, Li Z. Melatonin ameliorates renal fibroblast-myofibroblast transdifferentiation and renal fibrosis through miR-21-5p regulation. J Cell Mol Med. 2020;24:5615–28.
Ding Z, Wu X, Wang Y, Ji S, Zhang W, Kang J, et al. Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3beta/Nrf2 pathway. Biomed Pharmacother. 2020;132:110827.
Zhao X, Sun J, Su W, Shan H, Zhang B, Wang Y, et al. Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. Int J Mol Sci. 2018;19:1118. https://doi.org/10.3390/ijms19041118.
Gu X, Han YY, Yang CY, Ji HM, Lan YJ, Bi YQ, et al. Activated AMPK by metformin protects against fibroblast proliferation during pulmonary fibrosis by suppressing FOXM1. Pharmacol Res. 2021;173:105844.
Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, et al. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44:507–11.
Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–30.
Kasper M, Hughes RC. Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol. 1996;179:309–16.
Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–98.
Duecker R, Baer P, Eickmeier O, Strecker M, Kurz J, Schaible A, et al. Oxidative stress-driven pulmonary inflammation and fibrosis in a mouse model of human ataxia-telangiectasia. Redox Biol. 2018;14:645–55.
Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–21.
Sambo P, Baroni SS, Luchetti M, Paroncini P, Dusi S, Orlandini G, et al. Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001;44:2653–64.
Cho HY, Kleeberger SR. Noblesse oblige: NRF2 functions in the airways. Am J Respir Cell Mol Biol. 2014;50:844–7.
Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244:43–56.
Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016;11:3214–25.
Ahmadi Z, Ashrafizadeh M. Melatonin as a potential modulator of Nrf2. Fundam Clin Pharmacol. 2020;34:11–9.
Leon J, Acuna-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38:1–9.
Lopez LC, Escames G, Tapias V, Utrilla P, Leon J, Acuna-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol. 2006;38:267–78.
Kleber A, Kubulus D, Rossler D, Wolf B, Volk T, Speer T, et al. Melatonin modifies cellular stress in the liver of septic mice by reducing reactive oxygen species and increasing the unfolded protein response. Exp Mol Pathol. 2014;97:565–71.
Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060–5.
Ou SM, Tsai MT, Chen HY, Li FA, Tseng WC, Lee KH, et al. Identification of galectin-3 as potential biomarkers for renal fibrosis by RNA-sequencing and clinicopathologic findings of kidney biopsy. Front Med (Lausanne). 2021;8:748225.
Rajput VK, MacKinnon A, Mandal S, Collins P, Blanchard H, Leffler H, et al. A selective galactose-coumarin-derived galectin-3 inhibitor demonstrates involvement of galectin-3-glycan interactions in a pulmonary fibrosis model. J Med Chem. 2016;59:8141–7.
Jia W, Wang Z, Gao C, Wu J, Wu Q. Trajectory modeling of endothelial-to-mesenchymal transition reveals galectin-3 as a mediator in pulmonary fibrosis. Cell Death Dis. 2021;12:327.
Delaine T, Collins P, MacKinnon A, Sharma G, Stegmayr J, Rajput VK, et al. Galectin-3-binding glycomimetics that strongly reduce bleomycin-induced lung fibrosis and modulate intracellular glycan recognition. Chembiochem. 2016;17:1759–70.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Moghadam ZM, Henneke P, Kolter J. From flies to Men: ROS and the NADPH oxidase in phagocytes. Front Cell Dev Biol. 2021;9:628991.
He J, Li X, Luo H, Li T, Zhao L, Qi Q, et al. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens. 2017;11:275–89
Sun J, Zhang L, Fang J, Yang S, Chen L. Galectin-3 mediates high-glucose-induced cardiomyocyte injury by the NADPH oxidase/reactive oxygen species pathway. Can J Physiol Pharmacol. 2020;98:826–33.
Fenton-Navarro B, Garduno Rios D, Torner L, Letechipia-Vallejo G, Cervantes M. Melatonin decreases circulating levels of galectin-3 and cytokines, motor activity, and anxiety following acute global cerebral ischemia in male rats. Arch Med Res. 2021;52:505–13.
Novais AA, Chuffa LGA, Zuccari D, Reiter RJ. Exosomes and melatonin: where their destinies intersect. Front Immunol. 2021;12:692022.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81274172, 81473267 and 81973637); the National Traditional Chinese Medicine Inheritance and Innovation “Hundreds and Thousands” Talent Project: Young Qihuang Scholar Support Project of the State Administration of Traditional Chinese Medicine in 2020.
Author information
Authors and Affiliations
Contributions
YJL and JG conceived research project. YJL, HMJ, YQB, performed the experiments and data analysis. YYH, XG and CYY assisted the animal experiments. YJL, HLD, and MHC wrote the manuscript. HLD and JG supervised experiments and critically reviewed the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, Yj., Cheng, Mh., Ji, Hm. et al. Melatonin ameliorates bleomycin-induced pulmonary fibrosis via activating NRF2 and inhibiting galectin-3 expression. Acta Pharmacol Sin 44, 1029–1037 (2023). https://doi.org/10.1038/s41401-022-01018-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-01018-x
Keywords
This article is cited by
-
LGALS3 promotes liver fibrosis by enhancing the expression and phosphorylation of ERK1/2
European Journal of Medical Research (2025)
-
Inhibition of Galectin-3 by ADSC-derived exosomes after cavernous nerve injury ameliorates erectile dysfunction by modulating oxidative stress and inflammatory profibrotic cascades
Stem Cell Research & Therapy (2025)
-
Lysionotin attenuates bleomycin-induced pulmonary fibrosis by activating AMPK/Nrf2 pathway
Scientific Reports (2025)
-
Targeting AnxA2-EGFR signaling: hydroxychloroquine as a therapeutic strategy for bleomycin-induced pulmonary fibrosis
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
m6A reader IGF2BP1 facilitates macrophage glycolytic metabolism and fibrotic phenotype by stabilizing THBS1 mRNA to promote pulmonary fibrosis
Cellular and Molecular Life Sciences (2025)