Abstract
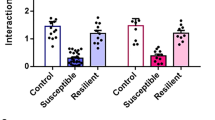
Emerging evidence demonstrates the vital role of synaptic transmission and structural remodeling in major depressive disorder. Activation of melanocortin receptors facilitates stress-induced emotional behavior. Prolylcarboxypeptidase (PRCP) is a serine protease, which splits the C-terminal amino acid of α-MSH and inactivates it. In this study, we asked whether PRCP, the endogenous enzyme of melanocortin system, might play a role in stress susceptibility via regulating synaptic adaptations. Mice were subjected to chronic social defeat stress (CSDS) or subthreshold social defeat stress (SSDS). Depressive-like behavior was assessed in SIT, SPT, TST and FST. Based on to behavioral assessments, mice were divided into the susceptible (SUS) and resilient (RES) groups. After social defeat stress, drug infusion or viral expression and behavioral tests, morphological and electrophysiological analysis were conducted in PFX-fixed and fresh brain slices containing the nucleus accumbens shell (NAcsh). We showed that PRCP was downregulated in NAcsh of susceptible mice. Administration of fluoxetine (20 mg·kg−1·d−1, i.p., for 2 weeks) ameliorated the depressive-like behavior, and restored the expression levels of PRCP in NAcsh of susceptible mice. Pharmacological or genetic inhibition of PRCP in NAcsh by microinjection of N-benzyloxycarbonyl-L-prolyl-L-prolinal (ZPP) or LV-shPRCP enhanced the excitatory synaptic transmission in NAcsh, facilitating stress susceptibility via central melanocortin receptors. On the contrary, overexpression of PRCP in NAcsh by microinjection of AAV-PRCP alleviated the depressive-like behavior and reversed the enhanced excitatory synaptic transmission, abnormal dendritogenesis and spinogenesis in NAcsh induced by chronic stress. Furthermore, chronic stress increased the level of CaMKIIα, a kinase closely related to synaptic plasticity, in NAcsh. The elevated level of CaMKIIα was reversed by overexpression of PRCP in NAcsh. Pharmacological inhibition of CaMKIIα in NAcsh alleviated stress susceptibility induced by PRCP knockdown. This study has revealed the essential role of PRCP in relieving stress susceptibility through melanocortin signaling-mediated synaptic plasticity in NAcsh.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–49.
Zhou HY, He JG, Hu ZL, Xue SG, Xu JF, Cui QQ, et al. A-kinase anchoring protein 150 and protein kinase A complex in the basolateral amygdala contributes to depressive-like behaviors induced by chronic restraint stress. Biol Psychiatry. 2019;86:131–42.
Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, et al. Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol Psychiatry. 2018;23:556–68.
McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–63.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902.
Castro DC, Bruchas MR. A motivational and neuropeptidergic hub: anatomical and functional diversity within the nucleus accumbens shell. Neuron. 2019;102:529–52.
Matikainen-Ankney BA, Kezunovic N, Menard C, Flanigan ME, Zhong Y, Russo SJ, et al. Parkinson’s disease-linked LRRK2-G2019S mutation alters synaptic plasticity and promotes resilience to chronic social stress in young adulthood. J Neurosci. 2018;38:9700–11.
Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–94.
Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–41.
Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–102.
Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, et al. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry. 2010;67:1075–82.
McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29.
Qu N, He Y, Wang C, Xu P, Yang Y, Cai X, et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatry. 2019;25:1006–21.
Diano S. New aspects of melanocortin signaling: a role for PRCP in alpha-MSH degradation. Front Neuroendocrinol. 2011;32:70–83.
Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–40.
Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK. Involvement of alpha-melanocyte stimulating hormone (alpha-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res. 2008;1216:53–67.
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9.
Jeong JK, Diano S. Prolyl carboxypeptidase and its inhibitors in metabolism. Trends Endocrinol Metab. 2013;24:61–67.
Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, et al. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest. 2009;119:2291–303.
Bruschetta G, Jin S, Liu ZW, Kim JD, Diano S. MC4R signaling in dorsal raphe nucleus controls feeding, anxiety, and depression. Cell Rep. 2020;33:108267.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol. 2020;177:3617–24.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–22.
Wu PF, Han QQ, Chen FF, Shen TT, Li YH, Cao Y, et al. Erasing m(6)A-dependent transcription signature of stress-sensitive genes triggers antidepressant actions. Neurobiol Stress. 2021;15:100390.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
Asano S, Komiya M, Koike N, Koga E, Nakatani S, Isobe Y. 5,6,7,8-Tetrahydropyrido[4,3-d]pyrimidines as novel class of potent and highly selective CaMKII inhibitors. Bioorg Med Chem Lett. 2010;20:6696–8.
Dumitriu D, Rodriguez A, Morrison JH. High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy. Nat Protoc. 2011;6:1391–411.
Chandra R, Engeln M, Schiefer C, Patton MH, Martin JA, Werner CT, et al. Drp1 mitochondrial fission in D1 neurons mediates behavioral and cellular plasticity during early cocaine abstinence. Neuron. 2017;96:1327–41.
Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–21.
Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, et al. Neuronal morphometry directly from bitmap images. Nat Methods. 2014;11:982–4.
Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27.
Churruca I, Portillo MP, Casis L, Gutierrez A, Macarulla MT, Echevarria E. Effects of fluoxetine administration on hypothalamic melanocortin system in obese Zucker rats. Neuropeptides. 2008;42:293–9.
Colombo G, Sordi A, Lonati C, Carlin A, Turcatti F, Leonardi P, et al. Treatment with alpha-melanocyte stimulating hormone preserves calcium regulatory proteins in rat heart allografts. Brain Behav Immun. 2008;22:817–23.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology. 2014;39:1178–86.
Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76:639–56.
Shen Y, Fu WY, Cheng EY, Fu AK, Ip NY. Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism. J Neurosci. 2013;33:464–72.
Caruso V, Lagerstrom MC, Olszewski PK, Fredriksson R, Schioth HB. Synaptic changes induced by melanocortin signalling. Nat Rev Neurosci. 2014;15:98–110.
Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–31.
Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–44.
Fox ME, Chandra R, Menken MS, Larkin EJ, Nam H, Engeln M, et al. Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol Psychiatry. 2020;25:1022–34.
Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–53.
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2.
Schaible EV, Steinstrasser A, Jahn-Eimermacher A, Luh C, Sebastiani A, Kornes F, et al. Single administration of tripeptide alpha-MSH(11-13) attenuates brain damage by reduced inflammation and apoptosis after experimental traumatic brain injury in mice. PLoS One. 2013;8:e71056.
Jeong JK, Diano S. Prolyl carboxypeptidase mRNA expression in the mouse brain. Brain Res. 2014;1542:85–92.
Yang HY, Erdos EG, Chiang TS. New enzymatic route for the inactivation of angiotensin. Nature. 1968;218:1224–6.
Kask A, Rago L, Korrovits P, Wikberg JE, Schioth HB. Evidence that orexigenic effects of melanocortin 4 receptor antagonist HS014 are mediated by neuropeptide Y. Biochem Biophys Res Commun. 1998;248:245–9.
Myung CS, Kim BT, Choi SH, Song GY, Lee SY, Jahng JW. Role of neuropeptide Y and proopiomelanocortin in fluoxetine-induced anorexia. Arch Pharm Res. 2005;28:716–21.
Sarkar S, Legradi G, Lechan RM. Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res. 2002;945:50–9.
Vecsernyes M, Biro E, Gardi J, Julesz J, Telegdy G. Involvement of endogenous corticotropin-releasing factor in mediation of neuroendocrine and behavioral effects to alpha-melanocyte-stimulating hormone. Endocr Res. 2000;26:347–56.
Kobayashi K, Ikeda Y, Haneda E, Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci. 2008;28:6272–80.
Klenowski PM, Shariff MR, Belmer A, Fogarty MJ, Mu EW, Bellingham MC, et al. Prolonged consumption of sucrose in a binge-like manner, alters the morphology of medium spiny neurons in the nucleus accumbens shell. Front Behav Neurosci. 2016;10:54.
Mychasiuk R, Muhammad A, Gibb R, Kolb B. Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Res. 2013;1499:53–60.
Francis TC, Chandra R, Gaynor A, Konkalmatt P, Metzbower SR, Evans B, et al. Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol Psychiatry. 2017;22:1512–9.
Kanjhan R, Noakes PG, Bellingham MC. Emerging roles of filopodia and dendritic spines in motoneuron plasticity during development and disease. Neural Plast. 2016;2016:3423267.
Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016;2016:8056370.
Schmidt H, Eilers J. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci. 2009;27:229–43.
Cui H, Mason BL, Lee C, Nishi A, Elmquist JK, Lutter M. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiol Behav. 2012;106:201–10.
Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron. 2008;59:902–13.
Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553:497–509.
Briz V, Hsu YT, Li Y, Lee E, Bi X, Baudry M. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. J Neurosci. 2013;33:4317–28.
Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–93.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 82130110 [to JGC], U21A20363 [to FW], 81872849 to [LHL] and 81721005 [to JGC]), the Foundation for National Key R&D Program of China (Grant No. SIT2030-Major project-2021ZD0202900 [to JGC]).
Author information
Authors and Affiliations
Contributions
QD wrote the paper and performed the most experiments. SQZ performed the Western blotting and confocal imaging. PFY and WTD participated in constructing CSDS models and behavioral test. JGC and FW designed the project and revised the manuscript. LHL conceived the project and designed the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, Q., Zhang, Sq., Yang, Pf. et al. α-MSH-catabolic enzyme prolylcarboxypeptidase in nucleus accumbens shell ameliorates stress susceptibility in mice through regulating synaptic plasticity. Acta Pharmacol Sin 44, 1576–1588 (2023). https://doi.org/10.1038/s41401-023-01074-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-023-01074-x
Keywords
This article is cited by
-
Costunolide normalizes neuroinflammation and improves neurogenesis deficits in a mouse model of depression through inhibiting microglial Akt/mTOR/NF-κB pathway
Acta Pharmacologica Sinica (2025)
-
Identification of novel biomarkers associated with immune infiltration in major depression disorder and atopic dermatitis
Archives of Dermatological Research (2025)