Abstract
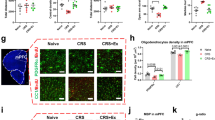
Exercise training effectively relieves anxiety disorders via modulating specific brain networks. The role of post-translational modification of proteins in this process, however, has been underappreciated. Here we performed a mouse study in which chronic restraint stress-induced anxiety-like behaviors can be attenuated by 14-day persistent treadmill exercise, in association with dramatic changes of protein phosphorylation patterns in the medial prefrontal cortex (mPFC). In particular, exercise was proposed to modulate the phosphorylation of Nogo-A protein, which drives the ras homolog family member A (RhoA)/ Rho-associated coiled-coil-containing protein kinases 1(ROCK1) signaling cascade. Further mechanistic studies found that liver-derived kynurenic acid (KYNA) can affect the kynurenine metabolism within the mPFC, to modulate this RhoA/ROCK1 pathway for conferring stress resilience. In sum, we proposed that circulating KYNA might mediate stress-induced anxiety-like behaviors via protein phosphorylation modification within the mPFC, and these findings shed more insights for the liver-brain communications in responding to both stress and physical exercise.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;19:Cd004691.
Singh B, Olds T, Curtis R, Dumuid D, Virgara R, Watson A, et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med. 2023;57:1203–9.
Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18:273–89.
Robbins JM, Gerszten RE. Exercise, exerkines, and cardiometabolic health: from individual players to a team sport. J Clin Invest. 2023;133:e168121.
Vints WAJ, Levin O, Fujiyama H, Verbunt J, Masiulis N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front Neuroendocrinol. 2022;66:100993.
Rody T, De Amorim JA, De Felice FG. The emerging neuroprotective roles of exerkines in Alzheimer’s disease. Front Aging Neurosci. 2022;14:965190.
Lee TH, Formolo DA, Kong T, Lau SW, Ho CS, Leung RYH, et al. Potential exerkines for physical exercise-elicited pro-cognitive effects: Insight from clinical and animal research. Int Rev Neurobiol. 2019;147:361–95.
Heo J, Noble EE, Call JA. The role of exerkines on brain mitochondria: a mini-review. J Appl Physiol (1985). 2023;134:28–35.
Yan L, Wei JA, Yang F, Wang M, Wang S, Cheng T, et al. Physical exercise prevented stress-induced anxiety via improving brain RNA methylation. Adv Sci (Weinh). 2022;9:e2105731.
Nestler EJ, Greengard P. Protein phosphorylation in the brain. Nature. 1983;305:583–8.
Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–82.
Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80.
Sánchez C, Díaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61:133–68.
Magnoni MS, Govoni S, Battaini F, Trabucchi M. The aging brain: protein phosphorylation as a target of changes in neuronal function. Life Sci. 1991;48:373–85.
Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21.
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–9.
Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–406.
Schmandke A, Schmandke A, Schwab ME. Nogo-A: multiple roles in CNS development, maintenance, and disease. Neuroscientist. 2014;20:372–86.
Xiao P, Gu J, Xu W, Niu X, Zhang J, Li J, et al. RTN4/Nogo-A-S1PR2 negatively regulates angiogenesis and secondary neural repair through enhancing vascular autophagy in the thalamus after cerebral cortical infarction. Autophagy. 2022;18:2711–30.
Xu W, Xiao P, Fan S, Chen Y, Huang W, Chen X, et al. Blockade of Nogo-A/Nogo-66 receptor 1 (NgR1) inhibits autophagic activation and prevents secondary neuronal damage in the thalamus after focal cerebral infarction in hypertensive rats. Neuroscience. 2020;431:103–14.
Zhou J, Ma Y, Chen J, Yao D, Feng C, Dong Y, et al. Effects of RhoA on depression-like behavior in prenatally stressed offspring rats. Behav Brain Res. 2022;432:113973.
Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794.
Yang YR, Kwon KS. Potential roles of exercise-induced plasma metabolites linking exercise to health benefits. Front Physiol. 2020;11:602748.
Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45.
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17.
Zhen D, Liu J, Zhang XD, Song Z. Kynurenic acid acts as a signaling molecule regulating energy expenditure and is closely associated with metabolic diseases. Front Endocrinol (Lausanne). 2022;13:847611.
Agudelo LZ, Ferreira DMS, Cervenka I, Bryzgalova G, Dadvar S, Jannig PR, et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27:378–392.e375.
Chen K, Zheng Y, Wei JA, Ouyang H, Huang X, Zhang F, et al. Exercise training improves motor skill learning via selective activation of mTOR. Sci Adv. 2019;5:eaaw1888.
Suo SB, Qiu JD, Shi SP, Chen X, Liang RP. PSEA: Kinase-specific prediction and analysis of human phosphorylation substrates. Sci Rep. 2014;4:4524.
Rees RW, Ralph DJ, Royle M, Moncada S, Cellek S. Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol. 2001;133:455–8.
Figlia G, Willnow P, Teleman AA. Metabolites regulate cell signaling and growth via covalent modification of proteins. Dev Cell. 2020;54:156–70.
Kocki T, Urbańska EM, Kocki J, Kloc R, Kocka K, Olajossy M, et al. Prolonged therapy with antidepressants increases hippocampal level of kynurenic acid and expression of Kat1 and Kat2 genes. Pharm Rep. 2018;70:737–45.
Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neural Transm (Vienna). 2012;119:235–43.
Yan Z, Yan H, Ou H. Human thyroxine binding globulin (TBG) promoter directs efficient and sustaining transgene expression in liver-specific pattern. Gene. 2012;506:289–94.
Pierozan P, Zamoner A, Soska AK, Silvestrin RB, Loureiro SO, Heimfarth L, et al. Acute intrastriatal administration of quinolinic acid provokes hyperphosphorylation of cytoskeletal intermediate filament proteins in astrocytes and neurons of rats. Exp Neurol. 2010;224:188–96.
Rissman RA. Stress-induced tau phosphorylation: functional neuroplasticity or neuronal vulnerability? J Alzheimers Dis. 2009;18:453–7.
Liu Y, Cao L, Zhang X, Liang Y, Xu Y, Zhu C. Memantine differentially regulates Tau phosphorylation induced by chronic restraint stress of varying duration in mice. Neural Plast. 2019;2019:4168472.
Yamamori S, Sugaya D, Iida Y, Kokubo H, Itakura M, Suzuki E, et al. Stress-induced phosphorylation of SNAP-25. Neurosci Lett. 2014;561:182–7.
Sabio G, Reuver S, Feijoo C, Hasegawa M, Thomas GM, Centeno F, et al. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem J. 2004;380:19–30.
Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99.
Kam TI, Park H, Chou SC, Van Vranken JG, Mittenbühler MJ, Kim H, et al. Amelioration of pathologic α-synuclein-induced Parkinson’s disease by irisin. Proc Natl Acad Sci USA. 2022;119:e2204835119.
Zhang SS, Zhu L, Peng Y, Zhang L, Chao FL, Jiang L, et al. Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice. J Neuroinflammation. 2022;19:34.
da Silva Rosa SC, Martens MD, Field JT, Nguyen L, Kereliuk SM, Hai Y, et al. BNIP3L/Nix-induced mitochondrial fission, mitophagy, and impaired myocyte glucose uptake are abrogated by PRKA/PKA phosphorylation. Autophagy. 2021;17:2257–72.
Wang T, Xiong JQ, Ren XB, Sun W. The role of Nogo-A in neuroregeneration: a review. Brain Res Bull. 2012;87:499–503.
Maldonado Bouchard S, Hook MA. Psychological stress as a modulator of functional recovery following spinal cord injury. Front Neurol. 2014;5:44.
Ruan Y, Cheng J, Dai J, Ma Z, Luo S, Yan R, et al. Chronic stress hinders sensory axon regeneration via impairing mitochondrial cristae and OXPHOS. Sci Adv. 2023;9:eadh0183.
Yokoyama K, Tezuka T, Hoshina N, Nakazawa T, Yamamoto T. Phosphorylation at Tyr-694 of Nogo-A by Src-family kinases. Biochem Biophys Res Commun. 2006;349:1401–5.
Babur Ö, Ngo ATP, Rigg RA, Pang J, Rub ZT, Buchanan AE, et al. Platelet procoagulant phenotype is modulated by a p38-MK2 axis that regulates RTN4/Nogo proximal to the endoplasmic reticulum: utility of pathway analysis. Am J Physiol Cell Physiol. 2018;314:C603–C615.
Glotfelty EJ, Tovar YRLB, Hsueh SC, Tweedie D, Li Y, Harvey BK, et al. The RhoA-ROCK1/ROCK2 pathway exacerbates inflammatory signaling in immortalized and primary microglia. Cells. 2023;12:1367.
Liu Q, Liu C, Lei B. siRNA mediated downregulation of RhoA expression reduces oxidative induced apoptosis in retinal ganglion cells. Curr Mol Med. 2023;24:630–6.
Fox ME, Chandra R, Menken MS, Larkin EJ, Nam H, Engeln M, et al. Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol Psychiatry. 2020;25:1022–34.
Sekine A, Fukuwatari T. Acute liver failure increases kynurenic acid production in rat brain via changes in tryptophan metabolism in the periphery. Neurosci Lett. 2019;701:14–19.
Olajossy M, Olajossy B, Wnuk S, Potembska E, Urbańska E. Blood serum concentrations of kynurenic acid in patients diagnosed with recurrent depressive disorder, depression in bipolar disorder, and schizoaffective disorder treated with electroconvulsive therapy. Psychiatr Pol. 2017;51:455–68.
Wu Y, Zhong X, Mai N, Wen Y, Shang D, Hu L, et al. Kynurenine pathway changes in late-life depression. J Affect Disord. 2018;235:76–81.
Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN, et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry. 2017;7:e1115.
Cogo A, Mangin G, Maïer B, Callebert J, Mazighi M, Chabriat H, et al. Increased serum QUIN/KYNA is a reliable biomarker of post-stroke cognitive decline. Mol Neurodegener. 2021;16:7.
Wu Y, Mai N, Zhong X, Wen Y, Zhou Y, Li H, et al. Kynurenine pathway changes in late-life depression with memory deficit. Psychiatry Res. 2018;269:45–49.
Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–22.
Acknowledgements
We thank Dr. Run-hua Wang (The Affiliated Brain Hospital of Guangzhou Medical University) for the help in collecting human blood samples. This work was funded by STI2030-Major Projects (2022ZD0207600) to LZ, National Key Research and Development Program of China (2020YFA0113600) to LZ, National Natural Science Foundation of China (U22A20301 to KFS, 32070955 to LZ), Key Research and Development Plan of Ningxia (2022BEG01004) to KFS, and Guangdong Basic and Applied Basic Research Foundation (2023B1515040015) to LZ.
Author information
Authors and Affiliations
Contributions
Conceptualization, LY and LZ; Methodology, LY, WJW, TC and LZ; Validation, LY and LZ; Formal analysis, LY, WW and TC; Investigation, LY, WJW, TC, Yang-ze Wang, DRY, Ya-jie Wang, and FZY; Resources, WJW, DRY. and FZY. Data Curation, LY and LZ; Writing - Original Draft, LZ and LY; Writing – Review & Editing, WJW, LZ and KFS; Visualization, LY and LZ; Supervision, KFS and LZ; Project Administration, LZ and KFS; Funding Acquisition, LZ and KFS.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, L., Wang, Wj., Cheng, T. et al. Hepatic kynurenic acid mediates phosphorylation of Nogo-A in the medial prefrontal cortex to regulate chronic stress-induced anxiety-like behaviors in mice. Acta Pharmacol Sin 45, 2032–2044 (2024). https://doi.org/10.1038/s41401-024-01302-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01302-y
Keywords
This article is cited by
-
Long-term electroacupuncture and repetitive transcranial magnetic stimulation differentially slow the progression of Alzheimer's disease in AppNL−G−F mice
Alzheimer's Research & Therapy (2025)
-
Long-term GABA supplementation mitigates anxiety by modulating complement and neuroinflammatory pathways
npj Science of Food (2025)