Abstracts
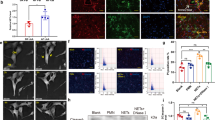
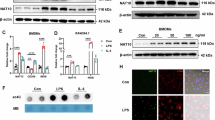
Cardiac fibrosis is a pathological scarring process that impairs cardiac function. N-acetyltransferase 10 (Nat10) is recently identified as the key enzyme for the N4-acetylcytidine (ac4C) modification of mRNAs. In this study, we investigated the role of Nat10 in cardiac fibrosis following myocardial infarction (MI) and the related mechanisms. MI was induced in mice by ligation of the left anterior descending coronary artery; cardiac function was assessed with echocardiography. We showed that both the mRNA and protein expression levels of Nat10 were significantly increased in the infarct zone and border zone 4 weeks post-MI, and the expression of Nat10 in cardiac fibroblasts was significantly higher compared with that in cardiomyocytes after MI. Fibroblast-specific overexpression of Nat10 promoted collagen deposition and induced cardiac systolic dysfunction post-MI in mice. Conversely, fibroblast-specific knockout of Nat10 markedly relieved cardiac function impairment and extracellular matrix remodeling following MI. We then conducted ac4C-RNA binding protein immunoprecipitation-sequencing (RIP-seq) in cardiac fibroblasts transfected with Nat10 siRNA, and revealed that angiomotin-like 1 (Amotl1), an upstream regulator of the Hippo signaling pathway, was the target gene of Nat10. We demonstrated that Nat10-mediated ac4C modification of Amotl1 increased its mRNA stability and translation in neonatal cardiac fibroblasts, thereby increasing the interaction of Amotl1 with yes-associated protein 1 (Yap) and facilitating Yap translocation into the nucleus. Intriguingly, silencing of Amotl1 or Yap, as well as treatment with verteporfin, a selective and potent Yap inhibitor, attenuated the Nat10 overexpression-induced proliferation of cardiac fibroblasts and prevented their differentiation into myofibroblasts in vitro. In conclusion, this study highlights Nat10 as a crucial regulator of myocardial fibrosis following MI injury through ac4C modification of upstream activators within the Hippo/Yap signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tallquist MD. Cardiac fibroblast diversity. Annu Rev Physiol. 2020;82:63–78.
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–40.
Pinto AR. Matricellular proteins as critical regulators of fibrosis. Circ Res. 2021;129:1036–8.
Stratton MS, Bagchi RA, Felisbino MB, Hirsch RA, Smith HE, Riching AS, et al. Dynamic chromatin targeting of BRD4 stimulates cardiac fibroblast activation. Circ Res. 2019;125:662–77.
Zhang QJ, Tran TAT, Wang M, Ranek MJ, Kokkonen-Simon KM, Gao J, et al. Histone lysine dimethyl-demethylase KDM3A controls pathological cardiac hypertrophy and fibrosis. Nat Commun. 2018;9:5230.
Mathiyalagan P, Adamiak M, Mayourian J, Sassi Y, Liang Y, Agarwal N, et al. FTO-dependent N(6)-methyladenosine regulates cardiac function during remodeling and repair. Circulation. 2019;139:518–32.
Stern L, Schulman LH. The role of the minor base N4-acetylcytidine in the function of the Escherichia coli noninitiator methionine transfer RNA. J Biol Chem. 1978;253:6132–9.
Dong C, Niu L, Song W, Xiong X, Zhang X, Zhang Z, et al. tRNA modification profiles of the fast-proliferating cancer cells. Biochem Biophys Res Commun. 2016;476:340–5.
Zhang H, Lu R, Huang J, Li L, Cao Y, Huang C, et al. N4-acetylcytidine modifies primary microRNAs for processing in cancer cells. Cell Mol Life Sci. 2024;81:73.
Dominissini D, Rechavi G. N(4)-acetylation of cytidine in mRNA by NAT10 regulates stability and translation. Cell. 2018;175:1725–7.
Sharma S, Langhendries JL, Watzinger P, Kotter P, Entian KD, Lafontaine DL. Yeast Kre33 and human NAT10 are conserved 18S rRNA cytosine acetyltransferases that modify tRNAs assisted by the adaptor Tan1/THUMPD1. Nucleic Acids Res. 2015;43:2242–58.
Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175:1872–86.e24.
Balmus G, Larrieu D, Barros AC, Collins C, Abrudan M, Demir M, et al. Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome. Nat Commun. 2018;9:1700.
Zhang Y, Jing Y, Wang Y, Tang J, Zhu X, Jin WL, et al. NAT10 promotes gastric cancer metastasis via N4-acetylated COL5A1. Signal Transduct Target Ther. 2021;6:173.
Yang W, Li HY, Wu YF, Mi RJ, Liu WZ, Shen X, et al. ac4C acetylation of RUNX2 catalyzed by NAT10 spurs osteogenesis of BMSCs and prevents ovariectomy-induced bone loss. Mol Ther Nucleic Acids. 2021;26:135–47.
Wei R, Cui X, Min J, Lin Z, Zhou Y, Guo M, et al. NAT10 promotes cell proliferation by acetylating CEP170 mRNA to enhance translation efficiency in multiple myeloma. Acta Pharm Sin B. 2022;12:3313–25.
Mia MM, Cibi DM, Binte Abdul Ghani SA, Singh A, Tee N, et al. Loss of Yap/taz in cardiac fibroblasts attenuates adverse remodeling and improves cardiac function. Cardiovasc Res. 2022;118:1785-804.
Mia MM, Cibi DM, Abdul Ghani SAB, Song W, Tee N, Ghosh S, et al. YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction. PLoS Biol. 2020;18:e3000941.
Li TY, Su W, Li LL, Zhao XG, Yang N, Gai JX, et al. Critical role of PAFR/YAP1 positive feedback loop in cardiac fibrosis. Acta Pharmacol Sin. 2022;43:2862–72.
Garoffolo G, Casaburo M, Amadeo F, Salvi M, Bernava G, Piacentini L, et al. Reduction of cardiac fibrosis by interference with YAP-dependent transactivation. Circ Res. 2022;131:239–57.
Fu D, Collins K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol Cell. 2007;28:773–85.
Tan TZ, Miow QH, Huang RY, Wong MK, Ye J, Lau JA, et al. Functional genomics identifies five distinct molecular subtypes with clinical relevance and pathways for growth control in epithelial ovarian cancer. EMBO Mol Med. 2013;5:1051–66.
Zhang H, Hou W, Wang HL, Liu HJ, Jia XY, Zheng XZ, et al. GSK-3beta-regulated N-acetyltransferase 10 is involved in colorectal cancer invasion. Clin Cancer Res. 2014;20:4717–29.
Ito S, Akamatsu Y, Noma A, Kimura S, Miyauchi K, Ikeuchi Y, et al. A single acetylation of 18 S rRNA is essential for biogenesis of the small ribosomal subunit in Saccharomyces cerevisiae. J Biol Chem. 2014;289:26201–12.
Li RH, Tian T, Ge QW, He XY, Shi CY, Li JH, et al. A phosphatidic acid-binding lncRNA SNHG9 facilitates LATS1 liquid-liquid phase separation to promote oncogenic YAP signaling. Cell Res. 2021;31:1088–105.
Landry NM, Rattan SG, Filomeno KL, Meier TW, Meier SC, Foran SJ, et al. SKI activates the Hippo pathway via LIMD1 to inhibit cardiac fibroblast activation. Basic Res Cardiol. 2021;116:25.
Wei Y, Yee PP, Liu Z, Zhang L, Guo H, Zheng H, et al. NEDD4L-mediated Merlin ubiquitination facilitates Hippo pathway activation. EMBO Rep. 2020;21:e50642.
Qiao K, Liu Y, Xu Z, Zhang H, Zhang H, Zhang C, et al. RNA m6A methylation promotes the formation of vasculogenic mimicry in hepatocellular carcinoma via Hippo pathway. Angiogenesis. 2021;24:83–96.
Gong R, Gao X, Liu Y, Shen Y, Jiang Z, Wang X, et al. Cyclin L1 controls cardiomyocyte proliferation and heart repair after injury. Signal Transduct Target Ther. 2023;8:243.
Zheng Y, Vertuani S, Nystrom S, Audebert S, Meijer I, Tegnebratt T, et al. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res. 2009;105:260–70.
Ragni CV, Diguet N, Le Garrec JF, Novotova M, Resende TP, Pop S, et al. Amotl1 mediates sequestration of the Hippo effector Yap1 downstream of Fat4 to restrict heart growth. Nat Commun. 2017;8:14582.
Zhou Y, Zhang J, Li H, Huang T, Wong CC, Wu F, et al. AMOTL1 enhances YAP1 stability and promotes YAP1-driven gastric oncogenesis. Oncogene. 2020;39:4375–89.
Xiao Y, Hill MC, Li L, Deshmukh V, Martin TJ, Wang J, et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev. 2019;33:1491–505.
Gibault F, Corvaisier M, Bailly F, Huet G, Melnyk P, Cotelle P. Non-photoinduced biological properties of verteporfin. Curr Med Chem. 2016;23:1171–84.
Francisco J, Zhang Y, Jeong JI, Mizushima W, Ikeda S, Ivessa A, et al. Blockade of fibroblast YAP attenuates cardiac fibrosis and dysfunction through MRTF-A inhibition. JACC Basic Transl Sci. 2020;5:931–45.
Feng J, Gou J, Jia J, Yi T, Cui T, Li Z. Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. Onco Targets Ther. 2016;9:5371–81.
Acknowledgements
The work was supported by Heilongjiang Touyan Innovation Team Program [B.Z.C], HMU Marshal Initiative Funding (HMUMIF-21018), and the National Natural Science Foundation of China [82272389/92168119/82100300/82000226].
Author information
Authors and Affiliations
Contributions
Y.L. and XXW conceived and designed the project. XXW drafted the manuscript and analyzed the data. XXW, YMZ, and QYZ, JXZ, DHY, ZZZ, XYJ, SNL, HYJ, HYC, YY, HY, HL, QMOY helped with the study data collection. ZWP, HHL, WC, NW, and YL revised this article critically for important intellectual content. BZC made a final decision on the manuscript. YL, BZC, WYM and XFG provided the funding. All authors have read and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Xx., Zhao, Ym., Zhang, Qy. et al. Acetylcytidine modification of Amotl1 by N-acetyltransferase 10 contributes to cardiac fibrotic expansion in mice after myocardial infarction. Acta Pharmacol Sin 45, 1425–1437 (2024). https://doi.org/10.1038/s41401-024-01306-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01306-8
Keywords
This article is cited by
-
NAT10 induces N4-acetylcytidine modification of AdipoR1-mediated mitochondrial biogenesis against endothelial-to-mesenchymal transition in hypertension
Molecular Medicine (2025)
-
NAT10 mediates TLR2 to promote podocyte senescence in adriamycin-induced nephropathy
Cell Death & Disease (2025)
-
The effects of YAP/TAZ in cardiomyocytes: a scoping review
Molecular Biology Reports (2025)