Abstract
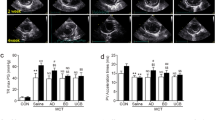
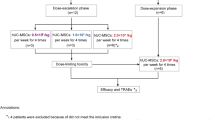
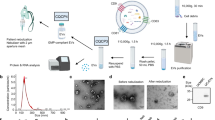
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have obvious advantages over MSC therapy. But the strong procoagulant properties of MSC-EVs pose a potential risk of thromboembolism, an issue that remains insufficiently explored. In this study, we systematically investigated the procoagulant activity of large EVs derived from human umbilical cord MSCs (UC-EVs) both in vitro and in vivo. UC-EVs were isolated from cell culture supernatants. Mice were injected with UC-EVs (0.125, 0.25, 0.5, 1, 2, 4 μg/g body weight) in 100 μL PBS via the tail vein. Behavior and mortality were monitored for 30 min after injection. We showed that these UC-EVs activated coagulation in a dose- and tissue factor-dependent manner. UC-EVs-induced coagulation in vitro could be inhibited by addition of tissue factor pathway inhibitor. Notably, intravenous administration of high doses of the UC-EVs (1 μg/g body weight or higher) led to rapid mortality due to multiple thrombus formations in lung tissue, platelets, and fibrinogen depletion, and prolonged prothrombin and activated partial thromboplastin times. Importantly, we demonstrated that pulmonary thromboembolism induced by the UC-EVs could be prevented by either reducing the infusion rate or by pre-injection of heparin, a known anticoagulant. In conclusion, this study elucidates the procoagulant characteristics and mechanisms of large UC-EVs, details the associated coagulation risk during intravenous delivery, sets a safe upper limit for intravenous dose, and offers effective strategies to prevent such mortal risks when high doses of large UC-EVs are needed for optimal therapeutic effects, with implications for the development and application of large UC-EV-based as well as other MSC-EV-based therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yoon YJ, Kim OY, Gho YS. Extracellular vesicles as emerging intercellular communicasomes. Bmb Rep. 2014;47:531–9.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404.
Skovronova R, Grange C, Dimuccio V, Deregibus MC, Camussi G, Bussolati B. Surface marker expression in small and medium/large mesenchymal stromal cell-derived extracellular vesicles in naive or apoptotic condition using orthogonal techniques. Cells. 2021;10:2948.
Zhang Q, Jeppesen DK, Higginbotham JN, Franklin JL, Coffey RJ. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat Protoc. 2023;18:1462–87.
Magoling BJA, Wu AY, Chen YJ, Wong WW, Chuo ST, Huang HC, et al. Membrane protein modification modulates big and small extracellular vesicle biodistribution and tumorigenic potential in breast cancers in vivo. Adv Mater. 2023;35:e2208966.
Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36:3012–28.
Kudo D, Goto T, Uchimido R, Hayakawa M, Yamakawa K, Abe T, et al. Coagulation phenotypes in sepsis and effects of recombinant human thrombomodulin: an analysis of three multicentre observational studies. Crit Care. 2021;25:114.
Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther. 2018;26:1610–23.
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–49.
Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346.
Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer’s disease. Int Immunol. 2017;29:11–9.
Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017;18:1450.
Lai P, Weng J, Guo L, Chen X, Du X. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019;7:6.
Chen Y, Tang Y, Fan GC, Duan DD. Extracellular vesicles as novel biomarkers and pharmaceutic targets of diseases. Acta Pharmacol Sin. 2018;39:499–500.
Song J, Song B, Yuan L, Yang G. Multiplexed strategies toward clinical translation of extracellular vesicles. Theranostics. 2022;12:6740–61.
Caivano A, Del Vecchio L, Musto P. Do we need to distinguish exosomes from microvesicles in hematological malignancies? Leukemia. 2017;31:2009–10.
Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4:743–53.
Guo L, Zhang Y, Wei R, Zhang X, Wang C, Feng M. Proinflammatory macrophage-derived microvesicles exhibit tumor tropism dependent on CCL2/CCR2 signaling axis and promote drug delivery via SNARE-mediated membrane fusion. Theranostics. 2020;10:6581–98.
Cai H, Guo H. Mesenchymal stem cells and their exocytotic vesicles. Int J Mol Sci. 2023;24:2085.
Rozier P, Maumus M, Maria ATJ, Toupet K, Lai-Kee-Him J, Jorgensen C, et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J Autoimmun. 2021;121:102660.
Du T, Zhou J, Chen WX, Zhang XL, Ji TY, Liu J, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells ameliorate renal ischemia-reperfusion injury via delivery of miR-21. Cell Cycle. 2020;19:1285–97.
Liang Z, Luo Y, Lv Y. Mesenchymal stem cell-derived microvesicles mediate BMP2 gene delivery and enhance bone regeneration. J Mater Chem B. 2020;8:6378–89.
Chen WX, Zhou J, Zhou SS, Zhang YD, Ji TY, Zhang XL, et al. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Res Ther. 2020;11:113.
Ye L, Song J, Zheng Y, Zhong M, Liu J, Zhu D, et al. New mechanism for mesenchymal stem cell microvesicle to restore lung permeability: intracellular S1P signaling pathway independent of S1P receptor-1. Stem Cell Res Ther. 2022;13:496.
Goradel NH, Jahangiri S, Negahdari B. Effects of mesenchymal stem cell-derived exosomes on angiogenesis in regenerative medicine. Curr Regenerat Med. 2018;7:46–53.
Fu FF, Zhu XJ, Wang HX, Zhang LM, Yuan GL, Chen ZC, et al. BCR-ABL1-positive microvesicles malignantly transform human bone marrow mesenchymal stem cells in vitro. Acta Pharmacol Sin. 2017;38:1475–85.
Zhang L, Lei Q, Wang H, Xu C, Liu T, Kong F, et al. Tumor-derived extracellular vesicles inhibit osteogenesis and exacerbate myeloma bone disease. Theranostics. 2019;9:196–209.
Liu Y, Zhu XJ, Zeng C, Wu PH, Wang HX, Chen ZC, et al. Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol Sin. 2014;35:230–8.
Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, et al. Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics. 2017;7:2673–89.
Lei Q, Gao F, Liu T, Ren W, Chen L, Cao Y, et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci Transl Med. 2021;13:eaaz8697.
Fiedler T, Rabe M, Mundkowski RG, Oehmcke-Hecht S, Peters K. Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int J Biochem Cell Biol. 2018;100:49–53.
Silachev DN, Goryunov KV, Shpilyuk MA, Beznoschenko OS, Morozova NY, Kraevaya EE, et al. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells. 2019;8:258.
Vasudevan M, Matsuura T, Chotani GK, Vieth WR. Membrane transport and biocatalytic reaction in an immobilized yeast membrane reactor. Ann N Y Acad Sci. 1987;506:345–56.
Wright A, Snyder O, He H, Christenson LK, Fleming S, Weiss ML. Procoagulant activity of umbilical cord-derived mesenchymal stromal cells’ extracellular vesicles (MSC-EVs). Int J Mol Sci. 2023;24:9216.
Yang B, Long Y, Zhang A, Wang H, Chen Z, Li Q. Procoagulant properties of mesenchymal stem cells and extracellular vesicles: a novel aspect of thrombosis pathogenesis. Stem Cells. 2024;42:98–106.
Chance TC, Rathbone CR, Kamucheka RM, Peltier GC, Cap AP, Bynum JA. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J Trauma Acute Care Surg. 2019;87:S74–S82.
Chen J, Ma Y, Wang Z, Wang H, Wang L, Xiao F, et al. Thrombin promotes fibronectin secretion by bone marrow mesenchymal stem cells via the protease-activated receptor mediated signalling pathways. Stem Cell Res Ther. 2014;5:36.
Kocaturk B, Versteeg HH. Tissue factor-integrin interactions in cancer and thrombosis: every Jack has his Jill. J Thromb Haemost. 2013;11:285–93.
Hisada Y, Sachetto ATA, Mackman N. Circulating tissue factor-positive extracellular vesicles and their association with thrombosis in different diseases. Immunol Rev. 2022;312:61–75.
Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70.
Thaler J, Ay C, Pabinger I. Clinical significance of circulating microparticles for venous thromboembolism in cancer patients. Hamostaseologie. 2012;32:127–31.
Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–80.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Gong L, Chen B, Zhang J, Sun Y, Yuan J, Niu X, et al. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J Extracell Vesicles. 2020;9:1800971.
Aass HC, Ovstebo R, Troseid AM, Kierulf P, Berg JP, Henriksson CE. Fluorescent particles in the antibody solution result in false TF- and CD14-positive microparticles in flow cytometric analysis. Cytom A. 2011;79:990–9.
Cao YL, Chen WL, Lei Q, Gao F, Ren WX, Chen L, et al. The transplantation of rapamycin-treated senescent human mesenchymal stem cells with enhanced proangiogenic activity promotes neovascularization and ischemic limb salvage in mice. Acta Pharmacol Sin. 2022;43:2885–94.
Liu S, Wei L, Zhang Y, Xu M, Wang C, Zhou J. Procoagulant activity and cellular origin of microparticles in human amniotic fluid. Thromb Res. 2014;133:645–51.
Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–53.
Xu Y, Yang Y, Zheng H, Huang C, Zhu X, Zhu Y, et al. Intracavernous injection of size-specific stem cell spheroids for neurogenic erectile dysfunction: Efficacy and risk versus single cells. EBioMedicine. 2020;52:102656.
Villa F, Quarto R, Tasso R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics. 2019;11:557.
Key NS. Analysis of tissue factor positive microparticles. Thromb Res. 2010;125:S42–5.
Broze GJ Jr., Girard TJ, Novotny WF. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry 1990;29:7539–46.
Sverdlov ED. Amedeo Avogadro’s cry: what is 1 microg of exosomes? Bioessays. 2012;34:873–5.
Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30:2221–31.
Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23:1262–73.
Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–30.
Gamperl H, Plattfaut C, Freund A, Quecke T, Theophil F, Gieseler F. Extracellular vesicles from malignant effusions induce tumor cell migration: inhibitory effect of LMWH tinzaparin. Cell Biol Int. 2016;40:1050–61.
Gupta D, Zickler AM, El Andaloussi S. Dosing extracellular vesicles. Adv Drug Deliv Rev. 2021;178:113961.
Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med. 2000;160:2429–36.
Tatsumi K, Ohashi K, Matsubara Y, Kohori A, Ohno T, Kakidachi H, et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431:203–9.
Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31:32–8.
Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringden O, Volk HD, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25:149–63.
Liao L, Shi B, Chang H, Su X, Zhang L, Bi C, et al. Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7:106–16.
Acknowledgements
This research was supported by grants from the National Key Research and Development Program of China (2021YFA1101500), the National Natural Science Foundation of China (81974221, 81974009, and 92049119), the Hubei Innovation Group Foundation (2022CFA019) and the General Program of Health Commission of Hubei Province (WJ2023M126). We would like to thank Shao-hua Zhang, Si-si Li, Na Li, Fan Hu, and Wen-long Huang from the Medical Sub-center, Analytical & Testing Center, Huazhong University of Science & Technology for their technical assistance.
Author information
Authors and Affiliations
Contributions
ZCC and QBL conceived the idea and directed the entire study. BLY and YYL performed most of the experiments. BLY and YYL drafted the paper, and QBL revised it. QL, FG, WXR, YLC, DW, LYX, JQ, HL, YLY, AYZ, SW, and HXW contributed to conducting research, refining ideas, and finalizing the manuscript. All authors have approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Bl., Long, Yy., Lei, Q. et al. Lethal pulmonary thromboembolism in mice induced by intravenous human umbilical cord mesenchymal stem cell-derived large extracellular vesicles in a dose- and tissue factor-dependent manner. Acta Pharmacol Sin 45, 2300–2312 (2024). https://doi.org/10.1038/s41401-024-01327-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01327-3
Keywords
This article is cited by
-
Mesenchymal stem cell-derived extracellular vesicles exert Th1-mediated anti-inflammatory effects via miR-146a/NF-κB pathway: comparison with dupilumab in a mouse model of atopic dermatitis
Stem Cell Research & Therapy (2025)
-
Extracellular particles: emerging insights into central nervous system diseases
Journal of Nanobiotechnology (2025)