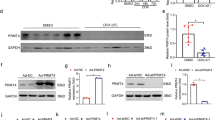
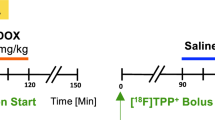
Abstract
Doxorubicin (DOX), a common chemotherapeutic agent in cancer therapy, is accompanied by pronounced cardiotoxicity. Ferroptosis has been implicated in the pathogenesis and therapeutics of DOX-induced cardiotoxicity (DIC). Asiatic acid (AA), a pentacyclic triterpene from the Chinese medicinal herb Centella asiatica, displays antioxidant, anti-inflammatory, and antiapoptotic activities. In this study, we investigated the beneficial effects of AA against DOX-induced ferroptosis and cardiotoxicity and the underlying mechanisms. A chronic DIC model was established by challenging mice with DOX (5 mg/kg, i.p.) once per week for 4 weeks. Concurrent with DOX insult, the mice were administered AA (25 mg·kg−1·d−1, i.g.). Cardiac function and mechanical properties of isolated cardiomyocytes were evaluated at the end of treatment. We showed that AA administration preserved cardiac function, significantly reduced cardiac injury, and improved cardiomyocyte contractile function in DIC mice. The beneficial effects of AA were causally linked to the inhibition of DOX-induced ferroptosis both in vivo and in vitro. We revealed that AA attenuated DOX-induced iron accumulation in HL-1 cells by increasing FPN-mediated iron export, in a Nrf2-dependent manner. AA upregulated Nrf2 expression and promoted Nrf2 nuclear translocation in DOX-treated HL-1 cells. Moreover, AA-offered benefits against DOX-induced cardiac dysfunction and ferroptosis were abolished by Nrf2 inhibitor ML385 (30 mg·kg-1·d-1, i.p.) administrated 30 min before AA in DIC mice. Our data favor that AA promotes FPN-mediated iron export to inhibit iron overload and ferroptosis in DIC, suggesting its therapeutic potential in the treatment of DIC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wu L, Wang L, Du Y, Zhang Y, Ren J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol Sci. 2023;44:34–49.
Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–801.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–79.
Wu L, Sowers JR, Zhang Y, Ren J. Targeting DNA damage response in cardiovascular diseases: from pathophysiology to therapeutic implications. Cardiovasc Res. 2023;119:691–709.
Yu W, Qin X, Zhang Y, Qiu P, Wang L, Zha W, et al. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther. 2020;10:752–69.
Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–80.
He H, Wang L, Qiao Y, Yang B, Yin D, He M. Epigallocatechin-3-gallate pretreatment alleviates doxorubicin-induced ferroptosis and cardiotoxicity by upregulating AMPKalpha2 and activating adaptive autophagy. Redox Biol. 2021;48:102185.
Hou K, Shen J, Yan J, Zhai C, Zhang J, Pan JA, et al. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine. 2021;69:103456.
Ta N, Qu C, Wu H, Zhang D, Sun T, Li Y, et al. Mitochondrial outer membrane protein FUNDC2 promotes ferroptosis and contributes to doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2022;119:e2117396119.
Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747.
Wang Y, Yan S, Liu X, Deng F, Wang P, Yang L, et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 2022;29:1982–95.
Wu L, Du Y, Wang L, Zhang Y, Ren J. Inhibition of METTL3 ameliorates doxorubicin-induced cardiotoxicity through suppression of TFRC-mediated ferroptosis. Redox Biol. 2024;72:103157.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82.
Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20:7–23.
Ma XH, Liu JH, Liu CY, Sun WY, Duan WJ, Wang G, et al. ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct Target Ther. 2022;7:288.
Jang S, Chapa-Dubocq XR, Tyurina YY, St Croix CM, Kapralov AA, Tyurin VA, et al. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021;45:102021.
Ito J, Omiya S, Rusu MC, Ueda H, Murakawa T, Tanada Y, et al. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. Elife. 2021;10:e62174.
Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12:708–22.
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303–18.
Fang M, Wan W, Li Q, Wan W, Long Y, Liu H, et al. Asiatic acid attenuates diabetic retinopathy through TLR4/MyD88/NF-kappaB p65 mediated modulation of microglia polarization. Life Sci. 2021;277:119567.
Maquart FX, Chastang F, Simeon A, Birembaut P, Gillery P, Wegrowski Y. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol. 1999;9:289–96.
Yi C, Song M, Sun L, Si L, Yu D, Li B, et al. Asiatic acid alleviates myocardial ischemia-reperfusion injury by inhibiting the ROS-mediated mitochondria-dependent apoptosis pathway. Oxid Med Cell Longev. 2022;2022:3267450.
Qiu F, Yuan Y, Luo W, Gong YS, Zhang ZM, Liu ZM, et al. Asiatic acid alleviates ischemic myocardial injury in mice by modulating mitophagy- and glycophagy-based energy metabolism. Acta Pharmacol Sin. 2022;43:1395–407.
Li H, Tian X, Ruan Y, Xing J, Meng Z. Asiatic acid alleviates Ang-II induced cardiac hypertrophy and fibrosis via miR-126/PIK3R2 signaling. Nutr Metab. 2021;18:71.
Meng Z, Li HY, Si CY, Liu YZ, Teng S. Asiatic acid inhibits cardiac fibrosis throughNrf2/HO-1 and TGF-beta1/Smads signaling pathways in spontaneous hypertension rats. Int Immunopharmacol. 2019;74:105712.
Qiu W, An S, Wang T, Li J, Yu B, Zeng Z, et al. Melatonin suppresses ferroptosis via activation of the Nrf2/HO-1 signaling pathway in the mouse model of sepsis-induced acute kidney injury. Int Immunopharmacol. 2022;112:109162.
Shi H, Gao Y, Dong Z, Yang J, Gao R, Li X, et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ Res. 2021;129:383–96.
Min J, Wu L, Liu Y, Song G, Deng Q, Jin W, et al. Empagliflozin attenuates trastuzumab-induced cardiotoxicity through suppression of DNA damage and ferroptosis. Life Sci. 2023;312:121207.
Jiang L, Wang J, Wang K, Wang H, Wu Q, Yang C, et al. RNF217 regulates iron homeostasis through its E3 ubiquitin ligase activity by modulating ferroportin degradation. Blood. 2021;138:689–705.
Pei Z, Liu Y, Liu S, Jin W, Luo Y, Sun M, et al. FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. Metabolism. 2021;122:154840.
Wu H, Liu Q, Shan X, Gao W, Chen Q. ATM orchestrates ferritinophagy and ferroptosis by phosphorylating NCOA4. Autophagy. 2023;19:2062–77.
Chen PH, Wu J, Ding CC, Lin CC, Pan S, Bossa N, et al. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2020;27:1008–22.
Ghigo A, Li M, Hirsch E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim Biophys Acta. 2016;1863:1916–25.
Eisenstein RS. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr. 2000;20:627–62.
Siegert I, Schodel J, Nairz M, Schatz V, Dettmer K, Dick C, et al. Ferritin-mediated iron sequestration stabilizes hypoxia-inducible factor-1alpha upon LPS activation in the presence of ample oxygen. Cell Rep. 2015;13:2048–55.
Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42.
Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. 2010;116:4657–64.
Chen PH, Smith TJ, Wu J, Siesser PF, Bisnett BJ, Khan F, et al. Glycosylation of KEAP1 links nutrient sensing to redox stress signaling. EMBO J. 2017;36:2233–50.
Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal. 2018;29:1756–73.
Chen Y, Zhang S, Wang X, Guo W, Wang L, Zhang D, et al. Disordered signaling governing ferroportin transcription favors breast cancer growth. Cell Signal. 2015;27:168–76.
Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295–317.
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617–30.
Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu SC, et al. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020;27:540–55.
Ramalho MJ, Bravo M, Loureiro JA, Lima J, Pereira MC. Transferrin-modified nanoparticles for targeted delivery of Asiatic acid to glioblastoma cells. Life Sci. 2022;296:120435.
Ji Y, Zhang X, Chen J, Song S, Fang S, Wang Z, et al. Asiatic acid attenuates tubular injury in diabetic kidney disease by regulating mitochondrial dynamics via the Nrf-2 pathway. Phytomedicine. 2023;109:154552.
Zou W, Zhang K, Qiu Z, Xin R, Wang G, Zhang J, et al. Asiatic acid and andrographolide reduce hippocampal injury through suppressing neuroinflammation caused by Salmonella typhimurium infection. Food Chem Toxicol. 2023;172:113584.
Ge C, Zhang S, Mu H, Zheng S, Tan Z, Huang X, et al. Emerging mechanisms and disease implications of ferroptosis: potential applications of natural products. Front Cell Dev Biol. 2021;9:774957.
Miranda CJ, Makui H, Soares RJ, Bilodeau M, Mui J, Vali H, et al. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood. 2003;102:2574–80.
Panjrath GS, Patel V, Valdiviezo CI, Narula N, Narula J, Jain D. Potentiation of Doxorubicin cardiotoxicity by iron loading in a rodent model. J Am Coll Cardiol. 2007;49:2457–64.
Cardoso S, Santos RX, Carvalho C, Correia S, Pereira GC, Pereira SS, et al. Doxorubicin increases the susceptibility of brain mitochondria to Ca2+-induced permeability transition and oxidative damage. Free Radic Biol Med. 2008;45:1395–402.
Pan J, Xiong W, Zhang A, Zhang H, Lin H, Gao L, et al. The imbalance of p53-Park7 signaling axis induces iron homeostasis dysfunction in doxorubicin-challenged cardiomyocytes. Adv Sci. 2023;10:e2206007.
Deng S, Yan T, Jendrny C, Nemecek A, Vincetic M, Godtel-Armbrust U, et al. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting both topoisomerase II isoforms. BMC Cancer. 2014;14:842.
Wallace KB, Sardao VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126:926–41.
Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62.
Oliveira T, Hermann E, Lin D, Chowanadisai W, Hull E, Montgomery M. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 2021;47:102149.
Kong N, Chen X, Feng J, Duan T, Liu S, Sun X, et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm Sin B. 2021;11:4045–54.
Wang P, Cui Y, Ren Q, Yan B, Zhao Y, Yu P, et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021;12:447.
Ito F, Kato K, Yanatori I, Murohara T, Toyokuni S. Ferroptosis-dependent extracellular vesicles from macrophage contribute to asbestos-induced mesothelial carcinogenesis through loading ferritin. Redox Biol. 2021;47:102174.
Li K, Chen B, Xu A, Shen J, Li K, Hao K, et al. TRIM7 modulates NCOA4-mediated ferritinophagy and ferroptosis in glioblastoma cells. Redox Biol. 2022;56:102451.
Fan J, Chen Q, Wei L, Zhou X, Wang R, Zhang H. Asiatic acid ameliorates CCl4-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-kappaB/IkappaBalpha, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther. 2018;12:3595–605.
Chen S, Huang Y, Su H, Zhu W, Wei Y, Long Y, et al. The integrated analysis of transcriptomics and metabolomics unveils the therapeutical effect of Asiatic acid on alcoholic hepatitis in rats. Inflammation. 2022;45:1780–99.
Zhao X, Tian Z, Sun M, Dong D. Nrf2: a dark horse in doxorubicin-induced cardiotoxicity. Cell Death Discov. 2023;9:261.
Yarmohammadi F, Rezaee R, Karimi G. Natural compounds against doxorubicin-induced cardiotoxicity: a review on the involvement of Nrf2/ARE signaling pathway. Phytother Res. 2021;35:1163–75.
Wang B, Jin Y, Liu J, Liu Q, Shen Y, Zuo S, et al. EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2. Redox Biol. 2023;65:102825.
Park JH, Seo YH, Jang JH, Jeong CH, Lee S, Park B. Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-κB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J Neuroinflammation. 2017;14:240.
Yang C, Guo Y, Huang TS, Zhao J, Huang XJ, Tang HX, et al. Asiatic acid protects against cisplatin-induced acute kidney injury via anti-apoptosis and anti-inflammation. Biomed Pharmacother. 2018;107:1354–62.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82130011, 92249301, and RFIS82250710173).
Author information
Authors and Affiliations
Contributions
LW and JR designed the study. LW, LTW, and YXD performed the study and analyzed the data. LW drafted the manuscript; YMZ and JR supervised the study and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, L., Wang, Lt., Du, Yx. et al. Asiatic acid ameliorates doxorubicin-induced cardiotoxicity by promoting FPN-mediated iron export and inhibiting ferroptosis. Acta Pharmacol Sin 46, 81–95 (2025). https://doi.org/10.1038/s41401-024-01367-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01367-9
Keywords
This article is cited by
-
Wogonin attenuates septic cardiomyopathy by suppressing ALOX15-mediated ferroptosis
Acta Pharmacologica Sinica (2025)