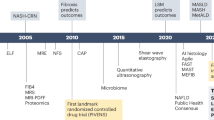
Abstract
Metabolic-associated liver disease is a growing public health crisis, with phenotypes from fatty liver to steatohepatitis, previously known as NASH (Non-Alcoholic SteatoHepatitis) and currently rebranded as MASH (Metabolic dysfunction-Associated SteatoHepatitis). Dysfunction in liver metabolism can progress to liver fibrosis, end stage cirrhosis and death. MASH (NASH) is associated with an increased risk of cardiovascular disease, elevation in serum lipids and Type 2 Diabetes Mellitus. There is now a US-approved drug to treat patients with NASH (MASH) under the FDA Accelerated Approval Pathway, which requires follow-up outcome studies to confirm clinical benefit or risk drug withdrawal by the agency. Despite extra-hepatic factors that contribute to MASH and complicate clinical trial design, reorganization of the FDA drug premarket review divisions, improvements to agency policies and procedures, as well as updates to the US Food, Drug & Cosmetic Act (FD&C Act) upon which FDA regulation is based, have provided new agency tools that facilitated such a drug approval to address the profound unmet medical need for patients with this metabolic-based liver disease. There is reason for hope that continued evolution of the regulatory process will lead to additional drug approvals, as well as the ability for clinical trial endpoints studying MASH treatments to include both liver-based and traditional metabolic measures, independent of the specific FDA review division. This initial NASH/MASH FDA approval has also opened the door for initiation of Combination Clinical Trials, where the approved drug is paired with an experimental drug with a different mechanism of action, to increase overall efficacy and potentially minimize risks. It is envisioned that future treatment of NASH/MASH will mirror what is currently observed with Type 2 Diabetes Mellitus practice patterns, where multiple drugs with different mechanisms of actions are used to optimize treatment benefit/risk in the selected patient populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ratziua V, Bellentanib S, Cortez-Pintoc H, Dayd C, Marchesinie G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84. https://www.journal-of-hepatology.eu/action/showPdf?pii=S0168-8278%2810%2900414-9
Rinella ME. Examining the nomenclature change from NAFLD and NASH to MASLD and MASH. Gastroenterol Hepatol (NY). 2023;19:697–9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10882866/pdf/GH_19_11_697.pdf
Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. https://doi.org/10.1002/hep.21327
Lichtenstein GR. Shifting our focus from NASH to MASH. Gastroenterol Hepatol (N. Y). 2023;19:511 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10524405/pdf/GH_19_9_511.pdf
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79 j:1542–56. https://www.journal-of-hepatology.eu/action/showPdf?pii=S0168-8278%2823%2900418-X December
American Association for the Study of Liver Diseases. New MASLD Nomenclature. 2023. https://www.aasld.org/new-masld-nomenclature
Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. https://www.nejm.org/doi/pdf/10.1056/NEJMra0912063?articleTools=true
Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol. 2020;6:9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7275502/pdf/40842_2020_Article_97.pdf
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. https://www.nejm.org/doi/pdf/10.1056/NEJMra1503519?articleTools=true
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. https://journals.lww.com/hep/fulltext/2018/01000/the_diagnosis_and_management_of_nonalcoholic_fatty.31.aspx
REZDIFFRA (resmetirom) tablets, for oral use Initial U.S. Approval: 14March 2024 (Drug Label) https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217785s000lbl.pdf
Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol Hepatol (N. Y). 2012;8:605–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594956/pdf/GH-08-605.pdf
Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. 2021;50:101167 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8324681/pdf/main.pdf
Zeng Y, He H, An Z. Advance of serum biomarkers and combined diagnostic panels in nonalcoholic fatty liver disease. Dis Markers. 2022;2022:1254014 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9259243/pdf/DM2022-1254014.pdf
A Brief History of the Center for Drug Evaluation and Research. 01/31/2018. https://www.fda.gov/about-fda/fda-history-exhibits/brief-history-center-drug-evaluation-and-research
FDA Center for Drug Evaluation Director-designate Carl Peck. PINK SHEET 05 Oct 1987. https://scrip.citeline.com/PS012562/FDA-Center-for-Drug-Evaluation-Director-designate-Carl-Peck
Drug and Biological Product Consolidation. Federal Register / Vol. 68, No. 123 / Thursday, June 26, 2003 / Notices. https://www.govinfo.gov/content/pkg/FR-2003-06-26/pdf/03-16242.pdf
FDA Office of New Drugs (OND) Annual Report (2022). https://www.fda.gov/media/172747/download?attachment
CFR - Code of Federal Regulations Title 21. INVESTIGATIONAL NEW DRUG APPLICATION. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?CFRPart=312
Personal Communication, Brian E Harvey MD PhD. March 17, 2024
Modernizing FDA’s New Drugs Regulatory Program. 12/05/2023 https://www.fda.gov/drugs/regulatory-science-research-and-education/modernizing-fdas-new-drugs-regulatory-program
Division of Hepatology and Nutrition (DHN). 08/11/2020. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-immunology-and-inflammation-division-hepatology-and-nutrition-dhn
Statement from FDA Commissioner Scott Gottlieb, M.D., on proposed modernization of FDA’s drug review office. June 04, 2018. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-proposed-modernization-fdas-drug-review-office
FDA Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics. May 2014. https://www.fda.gov/media/86377/download
Breakthrough Therapy Designation. 01/04/2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy
Fast Track. 01/04/2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track
Priority Review. 01/04/2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/priority-review
Accelerated Approval. 02/24/2023. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/accelerated-approval
Beakes-Read G, Neisser M, Frey P, Guarducci M. Analysis of FDA’s accelerated approval program performance december 1992–december 2021. Ther Innov Regul Sci. 2022;56:698–703. https://doi.org/10.1007/s43441-022-00430-z
Accelerated Approval Update under Food and Drug Omnibus Report Act of 2022 (FDORA). https://www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf
Final Decision on the Proposal to Withdraw Approval of Pepaxto (melphalan flufenamide) for Injection Docket No. FDA-2023-N-3167 February 23, 2024. https://www.fda.gov/media/176510/download?attachment
FDA issues final decision to withdraw approval of Pepaxto (melphalan flufenamide). 02/23/2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-issues-final-decision-withdraw-approval-pepaxto-melphalan-flufenamide
Endpoints and clinical trial design for nonalcoholic steatohepatitis. (AASLD 2009 Workshop Endpoints in Nonalcoholic Steatohepatitis). Hepatology. 2011;54:344–53. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4014460/pdf/nihms289374.pdf
FDA Guidance Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment (Draft). December 2018. https://www.fda.gov/media/119044/download
Filozof C, Chow SC, Dimick‐Santos L, Chen YF, Williams RN, Goldstein BJ, et al. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: Facilitating development approaches for an emerging epidemic. Hepatol Commun. 2017;1:577–85. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5721443/pdf/HEP4-1-577.pdf
Reorganization of the Office of New Drugs with Corresponding Changes to the Office of Translational Sciences and the Office of Pharmaceutical Quality. 05/21/2020. https://www.fda.gov/drugs/regulatory-science-research-and-education/reorganization-office-new-drugs-corresponding-changes-office-translational-sciences-and-office
Nonalcoholic Steatohepatitis with Compensated Cirrhosis: Developing Drugs for Treatment Guidance for Industry (Draft). June 2019. https://www.fda.gov/media/127738/download
Madrigal Pharmaceuticals Announces the Initiation of a Phase 2 Study of MGL-3196 in Patients with Non-alcoholic Steatohepatitis (NASH) October 20, 2016 https://ir.madrigalpharma.com/node/8641/pdf
NCT02912260. Phase 2 Study of MGL-3196 in Patients With Non-Alcoholic Steatohepatitis (NASH). https://classic.clinicaltrials.gov/ct2/show/NCT02912260?term=NCT02912260&draw=2&rank=1
Madrigal Pharmaceuticals Completes Enrollment in Phase 2 Proof-of-Concept Study with MGL-3196 for Treatment of NASH. August 21, 2017. https://ir.madrigalpharma.com/node/12566/pdf
Madrigal’s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial. May 31, 2018. https://ir.madrigalpharma.com/node/12791/pdf
Madrigal Pharmaceuticals Announces Publication in The Lancet of Positive Phase 2 Results for Resmetirom (MGL-3196) for the Treatment of Non-alcoholic Steatohepatitis (NASH). November 11, 2019. https://ir.madrigalpharma.com/node/13201/pdf
Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–24. https://www.natap.org/2022/HCV/PIIS01406736193251762.pdf Nov 30
Madrigal Pharmaceuticals Initiates Phase 3, Multinational, Double-Blind, Randomized, Placebo-Controlled Study of MGL-3196 (resmetirom) in Patients With Non-Alcoholic Steatohepatitis (NASH) and Fibrosis to Resolve NASH and Reduce Progression to Cirrhosis March 28, 2019. https://ir.madrigalpharma.com/node/13036/pdf
NCT03900429. A Phase 3 Study to Evaluate the Efficacy and Safety of MGL-3196 (Resmetirom) in Patients With NASH and Fibrosis (MAESTRO-NASH). https://classic.clinicaltrials.gov/ct2/show/NCT03900429?term=Resmetirom&draw=2&rank=4
Madrigal Pharmaceuticals Announces First Patient Dosed in MAESTRO-NAFLD-1, a Second Phase 3 Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study of Resmetirom (MGL-3196) in Patients With Non-Alcoholic Steatohepatitis (NASH) and Presumed NASH. December 18, 2019. https://ir.madrigalpharma.com/node/13251/pdf
NCT04197479. A Phase 3 Study to Evaluate Safety and Biomarkers of Resmetirom (MGL-3196) in Non Alcoholic Fatty Liver Disease Patients (MAESTRO-NAFLD1). https://classic.clinicaltrials.gov/ct2/show/NCT04197479?term=Resmetirom&draw=2&rank=1
Madrigal Pharmaceuticals Completes Enrollment of the 52 Week Liver Biopsy Patient Population in the Phase 3 MAESTRO-NASH Study of Resmetirom. June 30, 2021. https://ir.madrigalpharma.com/node/13726/pdf
Madrigal Pharmaceuticals Offers Patients Resmetirom in a Planned Open Label Active Treatment Extension of the Phase 3 MAESTRO-NAFLD-1 Clinical Study. July 13, 2021. https://ir.madrigalpharma.com/node/13736/pdf
NCT04951219. A Phase 3 Study to Evaluate Safety and Biomarkers of Resmetirom (MGL-3196) in Patients With Non-alcoholic Fatty Liver Disease (NAFLD), MAESTRO-NAFLD-Open-Label-Extension (MAESTRO-NAFLD-OLE). https://classic.clinicaltrials.gov/ct2/show/NCT04951219?term=Resmetirom&draw=2&rank=2
Positive Topline Phase 3 MAESTRO-NAFLD-1 Data Demonstrate Resmetirom was Safe, Well-Tolerated and Provided Statistically Significant Improvements in Key Measures of Liver and Cardiovascular Health. January 31, 2022. https://ir.madrigalpharma.com/node/14121/pdf
Madrigal Pharmaceuticals Initiates the MAESTRO-NASH Outcomes Study Evaluating Resmetirom for the Treatment of Patients with Compensated NASH Cirrhosis. August 31, 2022. https://ir.madrigalpharma.com/node/14471/pdf
NCT05500222. A Phase 3 Study to Evaluate the Effect of Resmetirom on Clinical Outcomes in Patients With Well-compensated NASH Cirrhosis (MAESTRO-NASH-OUTCOMES). https://classic.clinicaltrials.gov/ct2/show/NCT05500222?term=Resmetirom&draw=2&rank=3
Madrigal Announces Positive Topline Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH and Liver Fibrosis. December 19, 2022. https://ir.madrigalpharma.com/node/14516/pdf
Madrigal Receives Breakthrough Therapy Designation from FDA for Resmetirom and Completes Enrollment of the Phase 3 MAESTRO-NASH Biopsy Trial. April 18, 2023. https://ir.madrigalpharma.com/node/14701/pdf
Madrigal Pharmaceuticals Announces NDA Acceptance and Priority Review of the New Drug Application for Resmetirom for the Treatment of NASH with Liver Fibrosis. September 13, 2023. https://ir.madrigalpharma.com/node/14951/pdf
Madrigal Pharmaceuticals Announces Publication of the Phase 3 MAESTRO-NASH Trial of Resmetirom in the New England Journal of Medicine. February 8, 2024. https://ir.madrigalpharma.com/node/15291/pdf
Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497–509. https://doi.org/10.1056/NEJMoa2309000
Madrigal Pharmaceuticals Announces FDA Approval of Rezdiffra™ (resmetirom) for the Treatment of Patients with Noncirrhotic Nonalcoholic Steatohepatitis (NASH) with Moderate to Advanced Liver Fibrosis. March 14, 2024. https://ir.madrigalpharma.com/node/15401/pdf
FDA LETTER. NDA 217785 ACCELERATED APPROVAL. March 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/217785Orig1s000ltr.pdf
Division of Cardiology and Nephrology https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-cardiology-hematology-endocrinology-and-nephrology-ochen-division-cardiology-and-nephrology
Division of Diabetes, Lipid Disorders, and Obesity https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-cardiology-hematology-endocrinology-and-nephrology-division-diabetes-lipid-disorders-and
Office of Cardiology, Hematology, Endocrinology and Nephrology (OCHEN). https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-cardiology-hematology-endocrinology-and-nephrology-ochen
JARDIANCE® (empagliflozin tablets) Label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/204629s040lbl.pdf
FDA Jardiance Supplement Approval Letter (August 18, 2021) https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/204629Orig1s026ltr.pdf
FDA Jardiance Supplement Approval Letter (September 21, 2023) https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/204629Orig1s040ltr.pdf
Office of Immunology and Inflammation (OII). https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-immunology-and-inflammation-oii
Office of New Drugs (OND) “Super Office”. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-new-drugs
Anania FA, Dimick-Santos L, Mehta R, Toerner J, Beitz J. Nonalcoholic steatohepatitis: current thinking from the division of hepatology and nutrition at the food and drug administration. Hepatology. 2021;73:2023–7. https://doi.org/10.1002/hep.31687
Harvey BE. NASH: regulatory considerations for clinical drug development and U.S. FDA approval. Acta Pharmacol Sin. 2022;43:1210–4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9061714/pdf/41401_2021_Article_832.pdf
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harvey, B.E. How improvements in US FDA regulatory process and procedures led to the drug approval for first ever treatment of a common liver disease. Acta Pharmacol Sin 46, 515–524 (2025). https://doi.org/10.1038/s41401-024-01396-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01396-4