Abstract
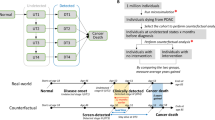
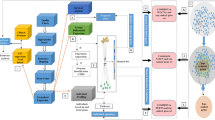
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers especially at advanced stage. In order to analyze the dynamics of potential prognostic biomarkers and further quantify their relationships with the overall survival (OS) of advanced PDAC patients, we herein developed a parametric time-to-event (TTE) model integrated with longitudinal submodels. Data from 104 patients receiving standard chemotherapies were retrospectively collected for model development, and other 54 patients were enrolled as external validation. The longitudinal submodels were developed with the time-course data of sum of longest diameters (SLD) of tumors, serum albumin (ALB) and body weight (BW) using nonlinear mixed effect models. The model-derived metrics including model parameters and individual predictions at different time points were further analyzed in the TTE model, together with other baseline information of patients. A linear growth-exponential shrinkage model was employed to describe the dynamics of SLD, while logistic models were used to fit the relationship of time prior to death with ALB and BW. The TTE model estimated the ALB and BW changes at the 9th week after chemotherapies as well as the baseline CA19-9 level that showed most significant impact on the OS, and the model-based simulations could provide individual survival rate predictions for patients with different prognostic factors. This study quantitatively demonstrates the importance of physical status and baseline disease for the OS of advanced PDAC patients, and highlights that timely nutrition support would be helpful to improve the prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493–502.
Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH. Survival and prognostic factors of unresectable pancreatic cancer. J Clin Gastroenterol. 2008;42:86–91.
Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, et al. The prognostic and predictive value of serum ca19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713–22.
Aliustaoglu M, Bilici A, Seker M, Dane F, Gocun M, Konya V, et al. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology. 2010;57:640–5.
Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014;20:10802–12.
Yao Y, Wang Z, Yong L, Yao Q, Tian X, Wang T, et al. Longitudinal and time-to-event modeling for prognostic implications of radical surgery in retroperitoneal sarcoma. CPT Pharmacomet Syst Pharmacol. 2022;11:1170–82.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Prim. 2018;4:17105.
Zheng Y, Narwal R, Jin C, Baverel PG, Jin X, Gupta A, et al. Population modeling of tumor kinetics and overall survival to identify prognostic and predictive biomarkers of efficacy for durvalumab in patients with urothelial carcinoma. Clin Pharmacol Ther. 2018;103:643–52.
Zecchin C, Gueorguieva I, Enas NH, Friberg LE. Models for change in tumour size, appearance of new lesions and survival probability in patients with advanced epithelial ovarian cancer. Br J Clin Pharmacol. 2016;82:717–27.
Terranova N, French J, Dai H, Wiens M, Khandelwal A, Ruiz-Garcia A, et al. Pharmacometric modeling and machine learning analyses of prognostic and predictive factors in the javelin gastric 100 phase iii trial of avelumab. CPT Pharmacomet Syst Pharmacol. 2022;11:333–47.
Garcia-Cremades M, Pitou C, Iversen PW, Troconiz IF. Predicting tumour growth and its impact on survival in gemcitabine-treated patients with advanced pancreatic cancer. Eur J Pharm Sci. 2018;115:296–303.
Wendling T, Mistry H, Ogungbenro K, Aarons L. Predicting survival of pancreatic cancer patients treated with gemcitabine using longitudinal tumour size data. Cancer Chemother Pharmacol. 2016;77:927–38.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wang Y, Sung C, Dartois C, Ramchandani R, Booth BP, Rock E, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167–74.
Sanghavi K, Ribbing J, Rogers JA, Ahmed MA, Karlsson MO, Holford N, et al. Covariate modeling in pharmacometrics: general points for consideration. CPT Pharmacomet Syst Pharmacol. 2024;13:710–28.
Holford N. A time to event tutorial for pharmacometricians. CPT Pharmacomet Syst Pharmacol. 2013;2:e43.
Gerds TA, Schumacher M. Efron-type measures of prediction error for survival analysis. Biometrics. 2007;63:1283–7.
Bruno R, Mercier F, Claret L. Evaluation of tumor size response metrics to predict survival in oncology clinical trials. Clin Pharmacol Ther. 2014;95:386–93.
Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol. 2015;16:e32–42.
Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11.
Lu Z, Fang Y, Liu C, Zhang X, Xin X, He Y, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39:748–56.
Lawrence Gould A, Boye ME, Crowther MJ, Ibrahim JG, Quartey G, Micallef S, et al. Joint modeling of survival and longitudinal non-survival data: current methods and issues. report of the DIA Bayesian joint modeling working group. Stat Med. 2015;34:2181–95.
Yoon SL, Kim JA, Kelly DL, Lyon D, George TJ Jr. Predicting unintentional weight loss in patients with gastrointestinal cancer. J Cachexia Sarcopenia Muscle. 2019;10:526–35.
Gilliland TM, Villafane-Ferriol N, Shah KP, Shah RM, Tran Cao HS, Massarweh NN, et al. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients. 2017;9:243.
Hendifar AE, Petzel MQB, Zimmers TA, Denlinger CS, Matrisian LM, Picozzi VJ, et al. Pancreas cancer-associated weight loss. Oncologist. 2019;24:691–701.
Poulia KA, Sarantis P, Antoniadou D, Koustas E, Papadimitropoulou A, Papavassiliou AG, et al. Pancreatic cancer and cachexia-metabolic mechanisms and novel insights. Nutrients. 2020;12:1543.
Liu XY, Zhang X, Ruan GT, Zhang KP, Tang M, Zhang Q, et al. One-year mortality in patients with cancer cachexia: association with albumin and total protein. Cancer Manag Res. 2021;13:6775–83.
Kim SH, Lee SM, Jeung HC, Lee IJ, Park JS, Song M, et al. The effect of nutrition intervention with oral nutritional supplements on pancreatic and bile duct cancer patients undergoing chemotherapy. Nutrients. 2019;11:1145.
Chung V, Sun V, Ruel N, Smith TJ, Ferrell BR. Improving palliative care and quality of life in pancreatic cancer patients. J Palliat Med. 2022;25:720–77.
Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, et al. Roles of ca19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875:188409.
Boyd LNC, Ali M, Comandatore A, Garajova I, Kam L, Puik JR, et al. Prediction model for early-stage pancreatic cancer using routinely measured blood biomarkers. JAMA Netw Open. 2023;6:e2331197.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2022YFF1203003) and Peking University Medicine Seed Fund for Interdisciplinary Research supported by the Fundamental Research Funds for the Central Universities (BMU2021MX003).
Author information
Authors and Affiliations
Contributions
TYZ, LS and QYY designed research; LXX, JSX and LY performed research; QYY, PYL, RC and JZ analyzed the data; QYY, PYL, TYZ and JZ wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Qy., Luo, Py., Xu, Lx. et al. Longitudinal and time-to-event modeling for the survival of advanced pancreatic ductal adenocarcinoma patients. Acta Pharmacol Sin 46, 751–758 (2025). https://doi.org/10.1038/s41401-024-01403-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01403-8
Keywords
This article is cited by
-
Quantitative systems pharmacology models: unleashing their potential in cancer immunotherapy
Journal of Pharmaceutical Investigation (2025)