Abstract
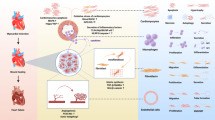
The current treatments and drugs of myocardial infarction (MI) remain insufficient. In recent years, natural products have garnered significant attention for their potential in treating cardiovascular diseases due to their availability and lower toxicity. Saponins, in particular, showed promising effects for cardiac protection. In this study, we investigated the therapeutic effects of the saponin compound madecassoside in the treatment of MI, and underlying molecular mechanisms. The acute MI model was established in male mice by ligation of the left anterior descending coronary artery. The mice were treated with madecassoside (20 mg· kg−1 ·d−1, i.g.) for 14 days. After sacrificing the mice, hearts were harvested for analysis. We showed that madecassoside administration significantly mitigated cardiac function decline in MI mice by promoting angiogenesis and inhibiting myocardial cell apoptosis and fibrosis. By conducting systems pharmacology and RNA sequencing, we demonstrated that madecassoside upregulated SPARC gene expression by activating protein kinase C-β (PKCB) that had a strong promoting effect on endothelial cell angiogenesis, thus playing a crucial protective role against MI. We showed that inhibition of SPARC gene significantly reduced madecassoside-stimulated migration and tube formation of endothelial cells in vitro; co-administration of the PKCB-specific inhibitor ruboxistaurin (10 mg· kg−1 ·d−1, i.g.) abolished the cardioprotective effect of madecassoside in MI mice, validating the critical role of the PKCB/SPARC signaling pathway. This study demonstrates that madecassoside regulates the PKCB/SPARC pathway, promotes the proliferation and regeneration of vascular endothelial cells, and effectively alleviates the symptoms of MI.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52.
Dani SS, Lone AN, Javed Z, Khan MS, Zia Khan M, Kaluski E, et al. Trends in premature mortality from acute myocardial infarction in the United States, 1999 to 2019. J Am Heart Assoc. 2022;11:e021682.
Khera R, Haimovich J, Hurley NC, McNamara R, Spertus JA, Desai N, et al. Use of machine learning models to predict death after acute myocardial infarction. JAMA Cardiol. 2021;6:633–41.
Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95.
Blue L, Kranker K, Markovitz AR, Powell RE, Williams MV, Pu J, et al. Effects of the million hearts model on myocardial infarctions, strokes, and medicare spending: a randomized clinical trial. JAMA. 2023;330:1437–47.
Stevens W, Peneva D, Li JZ, Liu LZ, Liu G, Gao R, et al. Estimating the future burden of cardiovascular disease and the value of lipid and blood pressure control therapies in China. BMC Health Serv Res. 2016;16:175.
Windecker S, Bax JJ, Myat A, Stone GW, Marber MS. Future treatment strategies in ST-segment elevation myocardial infarction. Lancet. 2013;382:644–57.
James SK, Spertus JA. Evidence-based treatments for STEMI: are we doing enough? Lancet. 2013;382:576–9.
Zhang Z, Zhao X, Gao M, Xu L, Qi Y, Wang J, et al. Dioscin alleviates myocardial infarction injury via regulating BMP4/NOX1-mediated oxidative stress and inflammation. Phytomedicine. 2022;103:154222.
Li H, Zhu J, Xu YW, Mou FF, Shan XL, Wang QL, et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 2022;54:102384.
Wang ZC, Niu KM, Wu YJ, Du KR, Qi LW, Zhou YB, et al. A dual Keap1 and p47(phox) inhibitor Ginsenoside Rb1 ameliorates high glucose/ox-LDL-induced endothelial cell injury and atherosclerosis. Cell Death Dis. 2022;13:824.
James J, Dubery I. Identification and quantification of triterpenoid centelloids in Centella asiatica (L.) Urban by densitometric TLC. J Planar Chromatogr-Mod TLC. 2011;24:82–7.
Hashim P, Sidek H, Helan MH, Sabery A, Palanisamy UD, Ilham M. Triterpene composition and bioactivities of Centella asiatica. Molecules. 2011;16:1310–22.
Choi SW, Cho W, Oh H, Abd El-Aty AM, Hong SA, Hong M, et al. Madecassoside ameliorates hepatic steatosis in high-fat diet-fed mice through AMPK/autophagy-mediated suppression of ER stress. Biochem Pharmacol. 2023;217:115815.
Bandopadhyay S, Mandal S, Ghorai M, Jha NK, Kumar M, Radha, et al. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: a review. J Cell Mol Med. 2023;27:593–608.
Bian GX, Li GG, Yang Y, Liu RT, Ren JP, Wen LQ, et al. Madecassoside reduces ischemia-reperfusion injury on regional ischemia induced heart infarction in rat. Biol Pharm Bull. 2008;31:458–63.
Bian D, Liu M, Li Y, Xia Y, Gong Z, Dai Y. Madecassoside, a triterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J Biochem Mol Toxicol. 2012;26:399–406.
Zhang P, Zhang D, Zhou W, Wang L, Wang B, Zhang T, et al. Network pharmacology: towards the artificial intelligence-based precision traditional Chinese medicine. Brief Bioinform. 2023;25:bbad518. https://doi.org/10.1093/bib/bbad518.
Li X, Liu Z, Liao J, Chen Q, Lu X, Fan X. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin J Nat Med. 2023;21:323–32.
Zhao L, Zhang H, Li N, Chen J, Xu H, Wang Y, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306.
Zhou J, Chen F, Yan A, Xia X. Madecassoside protects retinal pigment epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis through the activation of Nrf2/HO-1 pathway. Biosci Rep. 2020;40:BSR20194347.
Shan RR, Yu JT, Zhang SF, Xie MM, Hou R, Xie CY, et al. Madecassoside alleviates acute kidney injury by regulating JNK-mediated oxidative stress and programmed cell death. Phytomedicine. 2024;123:155252.
Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundbäck T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–22.
Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13.
Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–W64.
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45:W356–W60.
Luo H, Chen J, Shi L, Mikailov M, Zhu H, Wang K, et al. DRAR-CPI: a server for identifying drug repositioning potential and adverse drug reactions via the chemical-protein interactome. Nucleic Acids Res. 2011;39:W492–8.
Guo Q, Li Q, Liang W, Zhang Y, Jiang C, Zhang Y, et al. Asiatic acid and madecassic acid cause cardiotoxicity via inflammation and production of excessive reactive oxygen species in zebrafish. J Appl Toxicol. 2024;44:1028–39.
Junsai T, Tangpanithandee S, Srimangkornkaew P, Suknuntha K, Vivithanaporn P, Khemawoot P. Sub-chronic oral toxicity of a water-soluble extract of Centella asiatica (Centell-S) in Wistar rats. Food Chem Toxicol. 2024;185:114509.
Das SK, Yuan YF, Li MQ. Specific PKC βII inhibitor: one stone two birds in the treatment of diabetic foot ulcers. Biosci Rep. 2018;38:BSR20171459.
Wang F, Huang D, Zhu W, Li S, Yan M, Wei M, et al. Selective inhibition of PKCβ2 preserves cardiac function after myocardial infarction and is associated with improved angiogenesis of ischemic myocardium in diabetic rats. Int J Mol Med. 2013;32:1037–46.
Burkey JL, Campanale KM, Barbuch R, O’Bannon D, Rash J, Benson C, et al. Disposition of [14C]ruboxistaurin in humans. Drug Metab Dispos. 2006;34:1909–17.
Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, et al. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005;27:1164–80.
Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305:103–11.
Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–91.
Dorn GW 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–37.
Wang H, Xu Y, Xu A, Wang X, Cheng L, Lee S, et al. PKCβ/NF-κB pathway in diabetic atrial remodeling. J Physiol Biochem. 2020;76:637–53.
Muppala S, Frolova E, Xiao R, Krukovets I, Yoon S, Hoppe G, et al. Proangiogenic properties of thrombospondin-4. Arterioscler Thromb Vasc Biol. 2015;35:1975–86.
Deckx S, Johnson DM, Rienks M, Carai P, Van Deel E, Van der Velden J, et al. Extracellular SPARC increases cardiomyocyte contraction during health and disease. PLoS ONE. 2019;14:e0209534.
Zhao T, Wu W, Sui L, Huang Q, Nan Y, Liu J, et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47–72.
Sun Q, Ma H, Zhang J, You B, Gong X, Zhou X, et al. A self-sustaining antioxidant strategy for effective treatment of myocardial infarction. Adv Sci. 2023;10:e2204999.
Wang W, Wu L, Li Q, Zhang Z, Xu L, Lin C, et al. Madecassoside prevents acute liver failure in LPS/D-GalN-induced mice by inhibiting p38/NF-κB and activating Nrf2/HO-1 signaling. Biomed Pharmacother. 2018;103:1137–45.
Wei S, Chow LT, Shum IO, Qin L, Sanderson JE. Left and right ventricular collagen type I/III ratios and remodeling post-myocardial infarction. J Card Fail. 1999;5:117–26.
Lu L, Ying K, Wei S, Fang Y, Liu Y, Lin H, et al. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int J Dermatol. 2004;43:801–7.
Fan J, Zhang X, Jiang Y, Chen L, Sheng M, Chen Y. SPARC knockdown attenuated TGF-β1-induced fibrotic effects through Smad2/3 pathways in human pterygium fibroblasts. Arch Biochem Biophys. 2021;713:109049.
McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, et al. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;301:H497–505.
Luo X, Weng X, Bao X, Bai X, Lv Y, Zhang S, et al. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022;57:102511.
Wei S, Ma W, Jiang C, Liu J, Liu J, Zhang B, et al. Hyperoside prevents doxorubicin-induced cardiotoxicity by inhibiting NOXs/ROS/NLRP3 inflammasome signaling pathway. Phytother Res. 2023;37:4196–209.
Yuan H, Zhao Y, Li S, Qin J, Yu X. Madecassoside ameliorates cisplatin-induced nephrotoxicity by inhibiting activation of the mitogen activated protein kinase pathway. Environ Toxicol. 2023;38:1473–83.
Su Z, Ye J, Qin Z, Ding X. Protective effects of madecassoside against Doxorubicin induced nephrotoxicity in vivo and in vitro. Sci Rep. 2015;5:18314.
Peng LY, Shi HT, Yuan M, Li JH, Song K, Huang JN, et al. Madecassoside protects against LPS-induced acute lung injury via inhibiting TLR4/NF-κB activation and blood-air barrier permeability. Front Pharmacol. 2020;11:807.
Acknowledgements
This study was supported by Innovative Research Group Project of the National Natural Science Foundation of China (82121001); National Natural Science Foundation of China (32200973, 82270393); Natural Science Foundation of Jiangsu Province (BK20220310 to SL).
Author information
Authors and Affiliations
Contributions
SL and YJ contribute to conceptualization; PW and DDX contribute to methodology; PW contributes to software; SL, YJ, JQY, SJZ, and DDX contribute to validation; JQY contributes to formal analysis; SL, YJ, JQY, SJZ, and DDX contribute to investigation; SL and YJ contribute to resources; PW contributes to writing original draft; SL contributes to writing review and editing; PW and SL contribute to visualization; SL and YJ contribute to supervision; SL contributes to project administration; SL and YJ contribute to funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Yang, Jq., Xu, Dd. et al. Madecassoside mitigates acute myocardial infarction injury by activating the PKCB/SPARC signaling pathway. Acta Pharmacol Sin 46, 1624–1638 (2025). https://doi.org/10.1038/s41401-024-01442-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01442-1
Keywords
This article is cited by
-
Exploring the Role of SPARC in Atherosclerosis: Mechanisms and Potential Implications
Current Atherosclerosis Reports (2025)