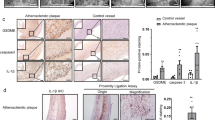
Abstract
The importance of ferroptosis in the occurrence and progression of atherosclerosis is gradually being recognized. The stimulatory G protein α subunit (Gsα) plays a crucial role in the physiology of endothelial cells (ECs). Our previous study showed that endothelial Gsα could regulate angiogenesis and preserve endothelial permeability. In this study, we investigated whether endothelial Gsα contributed to atherosclerosis through ferroptosis and oxidative stress. We generated endothelial Gsα-specific knockout mice in apolipoprotein E-deficient (ApoE−/−) background (ApoE−/−GsαECKO), and found that the mice exhibited aggravated atherosclerotic lesions and signs of ferroptosis compared with their wild-type littermates (ApoE−/−Gsαfl/fl). In human aortic endothelial cells (HAECs), overexpression of Gsα reduced lipid peroxidation and ferroptosis, whereas Gsα knockdown exacerbated oxidative stress and ferroptosis. Further, Gsα overexpression in HAECs increased the expression of antioxidant genes nuclear factor erythroid 2-related 2 (NRF2) and its downstream genes. Gsα regulated the expression of NRF2 through CCCTC-binding factor (CTCF). In conclusion, this study has revealed that Gsα acts as a defense factor against endothelial ferroptosis and is a potential target for the treatment of atherosclerosis and associated ischemic heart disease.

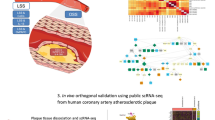
A model depicting the increase in the endothelial Gsα protein level in response to atherosclerotic stimuli. Gsα regulates NRF2 expression through cAMP/Epac/CTCF-mediated transcription and inhibits ferroptosis. Endothelial Gsα deficiency alleviates antioxidative stress and exacerbates atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–33.
Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122:S3–S14.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Prim. 2019;5:56.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25.
Yuan W, Xia H, Xu Y, Xu C, Chen N, Shao C, et al. The role of ferroptosis in endothelial cell dysfunction. Cell Cycle. 2022;21:1897–914.
Xu X, Xu XD, Ma MQ, Liang Y, Cai YB, Zhu ZX, et al. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed Pharmacother. 2024;171:116112.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82.
Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD, et al. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. 2019;59:555–75.
Xu P, Lin B, Deng X, Huang K, Zhang Y, Wang N. VDR activation attenuates osteoblastic ferroptosis and senescence by stimulating the Nrf2/GPX4 pathway in age-related osteoporosis. Free Radic Biol Med. 2022;193:720–35.
He F, Antonucci L, Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–16.
Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218.
Howden R. Nrf2 and cardiovascular defense. Oxid Med Cell Longev. 2013;2013:104308.
Alonso-Piñeiro JA, Gonzalez-Rovira A, Sánchez-Gomar I, Moreno JA, Durán-Ruiz MC. Nrf2 and heme oxygenase-1 involvement in atherosclerosis related oxidative stress. Antioxidants (Basel). 2021;10:1463.
Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, et al. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Am J Physiol Ren Physiol 2009;296:F1006–12.
Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–6.
Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest. 2019;129:2775–91.
Nakayama A, Albarrán-Juárez J, Liang G, Roquid KA, Iring A, Tonack S, et al. Disturbed flow-induced Gs-mediated signaling protects against endothelial inflammation and atherosclerosis. JCI insight. 2020;5:e140485.
He L, Lu H, Chu J, Qin X, Gao J, Chen M, et al. Endothelial G protein stimulatory α-subunit is a critical regulator of post-ischemic angiogenesis. Front Cardiovasc Med. 2022;9:941946.
He L, Lu H, Ji X, Chu J, Qin X, Chen M, et al. Stimulatory G-protein α subunit modulates endothelial cell permeability through regulation of plasmalemma vesicle-associated protein. Front Pharmacol. 2022;13:941064.
Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, et al. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest. 2005;115:3217–27.
Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–6.
Steiner T, Francescut L, Byrne S, Hughes T, Jayanthi A, Guschina I, et al. Protective role for properdin in progression of experimental murine atherosclerosis. PLoS One. 2014;9:e92404.
Li D, Mehta JL. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res. 2005;68:353–4.
Gan B. How erastin assassinates cells by ferroptosis revealed. Protein Cell. 2023;14:84–6.
Marques VB, Leal MAS, Mageski JGA, Fidelis HG, Nogueira BV, Vasquez EC, et al. Chronic iron overload intensifies atherosclerosis in apolipoprotein E deficient mice: Role of oxidative stress and endothelial dysfunction. Life Sci. 2019;233:116702.
Ouyang S, You J, Zhi C, Li P, Lin X, Tan X, et al. Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis. 2021;12:782.
Sun X, Niu X, Chen R, He W, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500.
Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107.
Zheng R, Dong X, Wan C, Shi X, Zhang X, Meyer C. Cistrome Data Browser and Toolkit: analyzing human and mouse genomic data using compendia of ChIP-seq and chromatin accessibility data. Quant Biol. 2020;8:267–76.
Kim S, Yu NK, Kaang BK. CTCF as a multifunctional protein in genome regulation and gene expression. Exp Mol Med. 2015;47:e166.
Shao X, Liu K, Fan Y, Ding Z, Chen M, Zhu M, et al. Gαs relays sphingosine-1-phosphate receptor 1 signaling to stabilize vascular endothelial-cadherin at endothelial junctions to control mouse embryonic vascular integrity. J Genet Genomics. 2015;42:613–24.
Qin X, He L, Tian M, Hu P, Yang J, Lu H, et al. Smooth muscle-specific Gsα deletion exaggerates angiotensin II-induced abdominal aortic aneurysm formation in mice in vivo. J Mol Cell Cardiol. 2019;132:49–59.
Qin X, Liu S, Lu Q, Zhang M, Jiang X, Hu S, et al. Heterotrimeric G stimulatory protein α subunit is required for intestinal smooth muscle contraction in mice. Gastroenterology. 2017;152:1114–25.e5.
Ma C, Li Y, Tian M, Deng Q, Qin X, Lu H, et al. Gsα regulates macrophage foam cell formation during atherosclerosis. Circ Res. 2024;134:e34–e51.
Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–8.
Aiken CT, Kaake RM, Wang X, Huang L. Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics. 2011;10:R110.006924. https://doi.org/10.1074/mcp.M110.006924.
Parnell E, Smith BO, Palmer TM, Terrin A, Zaccolo M, Yarwood SJ. Regulation of the inflammatory response of vascular endothelial cells by EPAC1. Br J Pharmacol. 2012;166:434–46.
Hilgendorf KI, Johnson CT, Mezger A, Rice SL, Norris AM, Demeter J, et al. Omega-3 fatty acids activate ciliary FFAR4 to control adipogenesis. Cell. 2019;179:1289–305.e21.
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96.
Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–8.
Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. 2018;76:713–22.
Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–8.
Nair S, Doh ST, Chan JY, Kong AN, Cai L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99:2070–82.
Erdos E, Sandor K, Young-Erdos CL, Halasz L, Smith SR, Osborne TF, et al. Transcriptional control of subcutaneous adipose tissue by the transcription factor CTCF modulates heterogeneity in fat distribution in women. Cells. 2023;13:86.
Bai T, Li M, Liu Y, Qiao Z, Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92–102.
Li L, Wang H, Zhang J, Chen X, Zhang Z, Li Q. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 2021;7:235.
Ji QX, Zeng FY, Zhou J, Wu WB, Wang XJ, Zhang Z, et al. Ferroptotic stress facilitates smooth muscle cell dedifferentiation in arterial remodelling by disrupting mitochondrial homeostasis. Cell Death Differ. 2023;30:457–74.
Jin R, Yang R, Cui C, Zhang H, Cai J, Geng B, et al. Ferroptosis due to cystathionine γ lyase/hydrogen sulfide downregulation under high hydrostatic pressure exacerbates VSMC dysfunction. Front Cell Dev Biol. 2022;10:829316.
Hu G, Yuan Z, Wang J. Autophagy inhibition and ferroptosis activation during atherosclerosis: Hypoxia-inducible factor 1α inhibitor PX-478 alleviates atherosclerosis by inducing autophagy and suppressing ferroptosis in macrophages. Biomed Pharmacother. 2023;161:114333.
Ko J, Jang S, Jang S, Park S, Yi J, Choi DK, et al. Glucose-dependent insulinotropic polypeptide (GIP) alleviates ferroptosis in aging-induced brain damage through the Epac/Rap1 signaling pathway. BMB Rep. 2024;57:417–23.
Boshchenko AA, Maslov LN, Mukhomedzyanov AV, Zhuravleva OA, Slidnevskaya AS, Naryzhnaya NV, et al. Peptides are cardioprotective drugs of the future: the receptor and signaling mechanisms of the cardioprotective effect of glucagon-like peptide-1 receptor agonists. Int J Mol Sci. 2024;25:4900.
Acknowledgements
The authors thank Prof. Yulong He from Soochow University for providing the Cdh5-CreERT2 mice.
Funding
This study was supported by grants from the Taishan Scholar Project of Shandong Province of China (No. tstp20240852), the Natural Science Foundation of Shandong Provincial (ZR2021QH112, ZR2024ZD23), the Natural Science Foundation for Distinguished Young Scolars of Shandong Province (ZR2020JQ30), the National Natural Science Foundation of China (No. 82100471, 82470499, 82270457), and the Postdoctoral Innovation Project of Shandong Province (SDCX-ZG-202400016).
Author information
Authors and Affiliations
Contributions
L-FH, LW, XTQ, WCZ, and CZ. designed the study. LFH, LW, J-WL, H-LL, XX, X-LY, P-DY, and J-GG, performed the experiments and analyzed the results. LFH, and WCZ drafted the manuscript. MC, F-PY, LSW, J-MY, JGG, CZ, and WCZ revised the manuscript for important intellectual content. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Lf., Wang, L., Li, Jw. et al. Endothelial Gsα deficiency promotes ferroptosis and exacerbates atherosclerosis in apolipoprotein E-deficient mice via the inhibition of NRF2 signaling. Acta Pharmacol Sin 46, 1289–1302 (2025). https://doi.org/10.1038/s41401-024-01446-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01446-x