Abstract
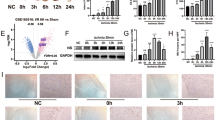
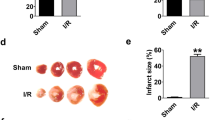
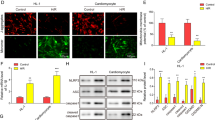
Eukaryotic translation initiation factor 4A1 (eIF4A1) is an ATP-dependent RNA helicase that participates in a variety of biological and pathological processes such as cell proliferation and apoptosis, and cancer. In this study we investigated the role of eIF4A1 in ischemic heart disease. The myocardial ischemia/reperfusion (I/R) model was established in mice by ligation of the left anterior descending artery for 45 min with the subsequent reperfusion for 24 h; cultured neonatal mouse ventricular cardiomyocytes (NMVCs) treated with H2O2 (200 μM) or H/R (12 h hypoxia and 12 h reoxygenation) were used for in vitro study. We showed that the expression levels of eIF4A1 were significantly increased in I/R-treated myocardium and in H2O2- or H/R-treated NMVCs. In NMVCs, eIF4A1 overexpression drastically enhanced LDH level, caspase 3 activity, and cell apoptosis. eIF4A1 overexpression also significantly reduced anti-apoptotic protein Bcl2 and elevated pro-apoptotic protein Bax expression, whereas eIF4A1 deficiency produced the opposite responses. Importantly, cardiomyocyte-specific eIF4A1 knockout attenuated cardiomyocyte apoptosis, reduced infarct area, and improved cardiac function in myocardial I/R mice. We demonstrated that eIF4A1 directly bound to transgelin (Tagln) to prevent its ubiquitination degradation and subsequent up-regulation of p53, and then promoted nuclear translocation of Tagln and p53. Nuclear localization of Tagln and p53 was increased in H2O2-treated NMVCs. Silencing Tagln reversed the pro-apoptotic effects of eIF4A1. Noticeably, eIF4A1 exerted the similar effects in AC16 human cardiomyocytes. In conclusion, eIF4A1 is a detrimental factor in myocardial I/R injury via promoting expression and nuclear translocation of Tagln and p53 and might be a potential target for myocardial I/R injury. This study highlights a novel biological role of eIF4A1 by interacting with non-translational-related factor Tagln in myocardial I/R injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rogers GW Jr., Komar AA, Merrick WC. eIF4A: the godfather of the DEAD box helicases. Prog Nucleic Acid Res Mol Biol. 2002;72:307–31.
Svitkin YV, Pause A, Haghighat A, Pyronnet S, Witherell G, Belsham GJ, et al. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5’ secondary structure. RNA. 2001;7:382–94.
Wang C, Leavenworth J, Zhang C, Liu Z, Yuan KY, Wang Y, et al. Epigenetic regulation of eIF4A1 through DNA methylation and an oncogenic role of eIF4A1 through BRD2 signaling in prostate cancer. Oncogene. 2022;41:2778–85.
Gao C, Guo X, Xue A, Ruan Y, Wang H, Gao X. High intratumoral expression of eIF4A1 promotes epithelial-to-mesenchymal transition and predicts unfavorable prognosis in gastric cancer. Acta Biochim Biophys Sin. 2020;52:310–9.
George J, Li Y, Kadamberi IP, Parashar D, Tsaih SW, Gupta P, et al. RNA-binding protein FXR1 drives cMYC translation by recruiting eIF4F complex to the translation start site. Cell Rep. 2021;37:109934.
Tyagi V, Parihar V, Singh D, Kapoor S, Kapoor M. The DEAD-box RNA helicase eIF4A1 interacts with the SWI2/SNF2-related chromatin remodelling ATPase DDM1 in the moss Physcomitrella. Biochim Biophys Acta Proteins Proteom. 2021;1869:140592.
Miao SB, Xie XL, Yin YJ, Zhao LL, Zhang F, Shu YN, et al. Accumulation of smooth muscle 22α protein accelerates senescence of vascular smooth muscle cells via stabilization of p53 in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2017;37:1849–59.
Chen Q, Zhang L, Wan C, Yang B, Kong X, Xu X, et al. Transgelin promotes ferroptosis to inhibit the malignant progression of esophageal squamous cell carcinoma. Int J Oncol. 2023;63:76
Zhang ZW, Yang ZM, Zheng YC, Chen ZD. Transgelin induces apoptosis of human prostate LNCaP cells through its interaction with p53. Asian J Androl. 2010;12:186–95.
Wei X, Lou H, Zhou D, Jia Y, Li H, Huang Q, et al. TAGLN mediated stiffness-regulated ovarian cancer progression via RhoA/ROCK pathway. J Exp Clin Cancer Res. 2021;40:292.
Li Y, Huang X, Jin J, Zhang H, Yang K, Han J, et al. Interaction of TAGLN and USP1 promotes ZEB1 ubiquitination degradation in UV-induced skin photoaging. Cell Biosci. 2023;13:80.
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–96.
Zhang XD, Qin ZH, Wang J. The role of p53 in cell metabolism. Acta Pharmacol Sin. 2010;31:1208–12.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–8.
Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–8.
Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–9.
Yang Y, Zhang Y, Yang J, Zhang M, Tian T, Jiang Y, et al. Interdependent nuclear co-trafficking of ASPP1 and p53 aggravates cardiac ischemia/reperfusion injury. Circ Res. 2023;132:208–22.
Liu PY, Tee AE, Milazzo G, Hannan KM, Maag J, Mondal S, et al. The long noncoding RNA lncNB1 promotes tumorigenesis by interacting with ribosomal protein RPL35. Nat Commun. 2019;10:5026.
Wu S, Xu S, Li R, Li K, Zhong X, Li Y, et al. mTORC1-Rps15 axis contributes to the mechanisms underlying global translation reduction during senescence of mouse embryonic fibroblasts. Front Cell Dev Biol. 2019;7:337.
Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature. 2014;513:65–70.
Modelska A, Turro E, Russell R, Beaton J, Sbarrato T, Spriggs K, et al. The malignant phenotype in breast cancer is driven by eIF4A1-mediated changes in the translational landscape. Cell Death Dis. 2015;6:e1603.
Liang S, Ju X, Zhou Y, Chen Y, Ke G, Wen H, et al. Downregulation of eukaryotic initiation factor 4A1 improves radiosensitivity by delaying DNA double strand break repair in cervical cancer. Oncol Lett. 2017;14:6976–82.
Ma X, Li B, Liu J, Fu Y, Luo Y. Phosphoglycerate dehydrogenase promotes pancreatic cancer development by interacting with eIF4A1 and eIF4E. J Exp Clin Cancer Res. 2019;38:66.
Wu C, Liu D, Zhang L, Wang J, Ding Y, Sun Z, et al. 5’-tiRNA-Gln inhibits hepatocellular carcinoma progression by repressing translation through the interaction with eukaryotic initiation factor 4A-I. Front Med. 2023;17:476-492.
Zhao Y, Wang Y, Chen W, Bai S, Peng W, Zheng M, et al. Targeted intervention of eIF4A1 inhibits EMT and metastasis of pancreatic cancer cells via c-MYC/miR-9 signaling. Cancer Cell Int. 2021;21:670.
Huang L, Li L, Yang T, Li W, Song L, Meng X, et al. Transgelin as a potential target in the reversibility of pulmonary arterial hypertension secondary to congenital heart disease. J Cell Mol Med. 2018;22:6249–61.
Chen Z, He S, Zhan Y, He A, Fang D, Gong Y, et al. TGF-beta-induced transgelin promotes bladder cancer metastasis by regulating epithelial-mesenchymal transition and invadopodia formation. EBioMedicine. 2019;47:208–20.
Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564:130–5.
Yang W, Zhuang Y, Wu H, Su S, Li Y, Wang C, et al. Substrate-dependent interaction of SPOP and RACK1 aggravates cardiac fibrosis following myocardial infarction. Cell Chem Biol. 2023;30:1248–60.e4
Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20:199–210.
Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309.
Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–34.
Seoane J, Le HV, Massagué J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–34.
Qi W, Li J, Pei X, Ke Y, Bu Q, Ni X β-Actin facilitates etoposide-induced p53 nuclear import. J Biosci. 2020;45:34.
Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, et al. Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem. 1998;273:29586–91.
Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–86.
Lain S, Lane D. Improving cancer therapy by non-genotoxic activation of p53. Eur J Cancer. 2003;39:1053–60.
Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science. 2001;292:1910–5.
Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604.
Eckerdt F, Yuan J, Saxena K, Martin B, Kappel S, Lindenau C, et al. Polo-like kinase 1-mediated phosphorylation stabilizes Pin1 by inhibiting its ubiquitination in human cells. J Biol Chem. 2005;280:36575–83.
Nihira NT, Ogura K, Shimizu K, North BJ, Zhang J, Gao D, et al. Acetylation-dependent regulation of MDM2 E3 ligase activity dictates its oncogenic function. Sci Signal. 2017;10:eaai8026.
Kim J, Chung IK. The splicing factor U2AF65 stabilizes TRF1 protein by inhibiting its ubiquitin-dependent proteolysis. Biochem Biophys Res Commun. 2014;443:1124–30.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82470285, 82070283, 82170393), National Key Research and Development Program of Ministry of Science and Technology (2023YFA1800902), and Haiyan Funding of Harbin Medical University Cancer Hospital (JJYQ2024-08).
Author information
Authors and Affiliations
Contributions
YJL, ZWP, YTZ, and DYL conceived and designed the research plan. DYL, XXH, ZRT, QWN, JQL, YY, WY, and BM contributed to data acquisition, data analysis, and data interpretation. JLL and YZ contributed to development or design of methodology. DYL, XXH, YTZ, YZ, YJL, and ZWP verified the data in the study. DYL, YTZ, YJL, and ZWP drafted the manuscript and all authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Dy., Hu, Xx., Tian, Zr. et al. eIF4A1 exacerbates myocardial ischemia-reperfusion injury in mice by promoting nuclear translocation of transgelin/p53. Acta Pharmacol Sin 46, 1236–1249 (2025). https://doi.org/10.1038/s41401-024-01467-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01467-6
Keywords
This article is cited by
-
Nanomedicine in cardiovascular and cerebrovascular diseases: targeted nanozyme therapies and their clinical potential and current challenges
Journal of Nanobiotechnology (2025)