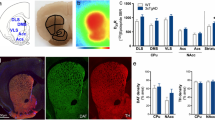
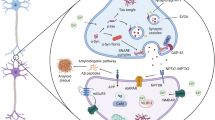
Abstract
Amyloid-beta (Aβ) aggregation, phosphorylated tau accumulation and neuroinflammation are considered as three hallmarks of Alzheimer’s disease (AD). Rhynchophylline (RN), the major alkaloid of a Chinese medicinal plant Uncaria rhynchophylla, has been shown to possess potent anti-AD effects. This study explored the effects of RN on Aβ pathology, tauopathy, and neuroinflammation using three AD mouse models, including TgCRND8, 3×Tg-AD, and 5×FAD, with RN treatment lasting for 4, 6, and 6 months, respectively, followed by behavioral tests and biological assays. In addition, BV2 cells were employed to further evaluate the biological effects of RN. RN treatment improved cognitive functions by reducing anxiety-like behaviors, enhancing recognition ability, and ameliorating learning impairments. It modulated Aβ processing through reducing the Aβ-producing enzyme activities and enhancing degradation enzyme activities, thereby diminishing Aβ accumulation. RN also decreased hyperphosphorylated tau proteins at Thr181, Thr205, Ser396, and Ser404 sites. Moreover, RN diminished neuroinflammation by reducing microglia and astrocyte activation and lowering the release of inflammatory cytokines. Furthermore, RN treatment could restore gut microbiota dysbiosis in 5×FAD mice. In BV2 cells, knockdown of p53, HDAC2, and Galectin-3 markedly enhanced the anti-inflammatory effects of RN. Overall, the anti-AD properties of RN were attributed to its regulation of multiple biological pathways, including regulation of the p53/PINK1 signaling pathway, inhibition of the HDAC2/AMPK signaling pathway, suppression of the Galectin-3/C/EBPβ/AEP signaling pathway, and modulation of gut microflora dysbiosis. This pioneering study unambiguously revealed the effects of RN on cognitive impairments, APP processing, tauopathy, and neuroinflammation in different transgenic mouse models with differing AD burdens, highlighting its potential as an anti-AD therapeutic agent and enhancing the scientific basis for its clinical use in treating AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
All data supporting the conclusions of this article are included with this article.
References
Iyaswamy A, Wang X, Zhang H, Vasudevan K, Wankhar D, Lu K, et al. Molecular engineering of a theranostic molecule that detects Aβ plaques, inhibits Iowa and Dutch mutation Aβ self-aggregation and promotes lysosomal biogenesis for Alzheimer’s disease. J Mater Chem B. 2024;12:7543–56.
Iyaswamy A, Wang X, Krishnamoorthi S, Kaliamoorthy V, Sreenivasmurthy SG, Kumar Durairajan SS, et al. Theranostic F-SLOH mitigates Alzheimer’s disease pathology involving TFEB and ameliorates cognitive functions in Alzheimer’s disease models. Redox Biol. 2022;51:102280.
Guan XJ, Deng ZQ, Liu J, Su CF, Tong BC, Zhu Z, et al. Corynoxine promotes TFEB/TFE3-mediated autophagy and alleviates Aβ pathology in Alzheimer’s disease models. Acta Pharmacol Sin. 2024;45:900–13.
Krishnamoorthi S, Iyaswamy A, Sreenivasmurthy SG, Thakur A, Vasudevan K, Kumar G, et al. PPARɑ ligand caudatin improves cognitive functions and mitigates Alzheimer’s disease defects by inducing autophagy in mice models. J Neuroimmune Pharmacol. 2023;18:509–28.
Iyaswamy A, Krishnamoorthi SK, Zhang H, Sreenivasmurthy SG, Zhu Z, Liu J, et al. Qingyangshen mitigates amyloid-β and Tau aggregate defects involving PPARα-TFEB activation in transgenic mice of Alzheimer’s disease. Phytomedicine. 2021;91:153648.
Tan W, Qi L, Hu X, Tan Z. Research progress in traditional Chinese medicine in the treatment of Alzheimer’s disease and related dementias. Front Pharmacol. 2022;13:921794.
Griciuc A, Tanzi RE. The role of innate immune genes in Alzheimer’s disease. Curr Opin Neurol. 2021;34:228–36.
Iyaswamy A, Thakur A, Guan XJ, Krishnamoorthi S, Fung TY, Lu K, et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct Target Ther. 2023;8:404.
Sun ZK, Yang HQ, Chen SD. Traditional Chinese medicine: a promising candidate for the treatment of Alzheimer’s disease. Transl Neurodegener. 2013;2:6.
Jiang P, Chen L, Xu J, Liu W, Feng F, Qu W. Neuroprotective effects of rhynchophylline against Aβ1-42-induced oxidative stress, neurodegeneration, and memory impairment via Nrf2-ARE activation. Neurochem Res. 2021;46:2439–50.
Xu QQ, Shaw PC, Hu Z, Yang W, Ip SP, Xian YF, et al. Comparison of the chemical constituents and anti-Alzheimer’s disease effects of Uncaria rhynchophylla and Uncaria tomentosa. Chin Med. 2021;16:110.
Xian YF, Lin ZX, Mao QQ, Hu Z, Zhao M, Che CT, et al. Bioassay-guided isolation of neuroprotective compounds from Uncaria rhynchophylla against beta-amyloid-induced neurotoxicity. Evid Based Complement Altern Med. 2012;2012:802625.
Li HQ, Ip SP, Yuan QJ, Zheng GQ, Tsim KKW, Dong TTX, et al. Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: Studies in a transgenic mouse model of Alzheimer’s disease. Brain Behav Immun. 2019;82:264–78.
Zhang C, Wu X, Xian Y, Zhu L, Lin G, Lin ZX. Evidence on integrating pharmacokinetics to find truly therapeutic agent for Alzheimer’s disease: comparative pharmacokinetics and disposition kinetics profiles of stereoisomers isorhynchophylline and rhynchophylline in rats. Evid Based Complement Altern Med. 2019;2019:4016323.
Hu S, Mak S, Zuo X, Li H, Wang Y, Han Y. Neuroprotection against MPP+-induced cytotoxicity through the activation of PI3K/Akt/GSK3β/MEF2D signaling pathway by rhynchophylline, the major tetracyclic oxindole alkaloid isolated from Uncaria rhynchophylla. Front Pharmacol. 2018;9:768.
Li H, Bi Q, Cui H, Lv C, Wang M. Suppression of autophagy through JAK2/STAT3 contributes to the therapeutic action of rhynchophylline on asthma. BMC Complement Med Ther. 2021;21:21.
Zhou J, Zhou S. Antihypertensive and neuroprotective activities of rhynchophylline: the role of rhynchophylline in neurotransmission and ion channel activity. J Ethnopharmacol. 2010;132:15–27.
Wang L, Wang Y, Chen Y, Liu B, Chou D, Bian X, et al. Rhynchophylline ameliorates cerebral ischemia by improving the synaptic plasticity in a middle cerebral artery occlusion induced stroke model. Eur J Pharmacol. 2023;940:175390.
Xu R, Wang J, Xu J, Song X, Huang H, Feng Y, et al. Rhynchophylline loaded-mPEG-PLGA nanoparticles coated with tween-80 for preliminary study in Alzheimer’s disease. Int J Nanomed. 2020;15:1149–60.
Shao H, Mi Z, Ji WG, Zhang CH, Zhang T, Ren SC, et al. Rhynchophylline protects against the amyloid β-induced increase of spontaneous discharges in the hippocampal CA1 region of rats. Neurochem Res. 2015;40:2365–73.
Fu AK, Hung KW, Huang H, Gu S, Shen Y, Cheng EY, et al. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proc Natl Acad Sci USA. 2014;111:9959–64.
Fu WY, Hung KW, Lau SF, Butt B, Yuen VW, Fu G, et al. Rhynchophylline administration ameliorates amyloid-β pathology and inflammation in an Alzheimer’s disease transgenic mouse model. ACS Chem Neurosci. 2021;12:4249–56.
Xu QQ, Su ZR, Yang W, Zhong M, Xian YF, Lin ZX. Patchouli alcohol attenuates the cognitive deficits in a transgenic mouse model of Alzheimer’s disease via modulating neuropathology and gut microbiota through suppressing C/EBPβ/AEP pathway. J Neuroinflammation. 2023;20:19.
López-Gambero AJ, Pacheco-Sánchez B, Rosell-Valle C, Medina-Vera D, Navarro JA, Fernández-Arjona MDM, et al. Dietary administration of D-chiro-inositol attenuates sex-specific metabolic imbalances in the 5×FAD mouse model of Alzheimer’s disease. Biomed Pharmacother. 2022;150:112994.
Belfiore R, Rodin A, Ferreira E, Velazquez R, Branca C, Caccamo A, et al. Temporal and regional progression of Alzheimer’s disease-like pathology in 3×Tg-AD mice. Aging Cell. 2019;18:e12873.
Hongyan L, Mengjiao Z, Chunyan W, Yaruo H. Rhynchophylline attenuates neurotoxicity in Tourette syndrome rats. Neurotox Res. 2019;36:679–87.
Liu J, Zhao Y, Zhu Y, Wang Y, Liu X, Nie X, et al. Rhynchophylline regulates calcium homeostasis by antagonizing ryanodine receptor 2 phosphorylation to improve diabetic cardiomyopathy. Front Pharmacol. 2022;13:882198.
Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58.
Zhang Z, Song M, Liu X, Kang SS, Kwon IS, Duong DM, et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat Med. 2014;20:1254–62.
Chen L, Huang C, Shentu J, Wang M, Yan S, Zhou F, et al. Indirubin derivative 7-bromoindirubin-3-oxime (7Bio) attenuates Aβ oligomer-induced cognitive impairments in mice. Front Mol Neurosci. 2017;10:393.
Wang X, Zheng M, Liu J, Huang Z, Bai Y, Ren Z, et al. Differences of first-pass effect in the liver and intestine contribute to the stereoselective pharmacokinetics of rhynchophylline and isorhynchophylline epimers in rats. J Ethnopharmacol. 2017;209:175–83.
Zhang T, Chen D, Lee TH. Phosphorylation signaling in APP processing in Alzheimer’s disease. Int J Mol Sci. 2019;21:209.
Pairojana T, Phasuk S, Suresh P, Huang SP, Pakaprot N, Chompoopong S, et al. Age and gender differences for the behavioral phenotypes of 3×Tg alzheimer’s disease mice. Brain Res. 2021;1762:147437.
Checler F, Goiran T, Alves da Costa C. Nuclear TP53: an unraveled function as transcriptional repressor of PINK1. Autophagy. 2018;14:1099–101.
Hooper C, Meimaridou E, Tavassoli M, Melino G, Lovestone S, Killick R. p53 is upregulated in Alzheimer’s disease and induces tau phosphorylation in HEK293a cells. Neurosci Lett. 2007;418:34–7.
Tan Y, Zheng Y, Xu D, Sun Z, Yang H, Yin Q. Galectin-3: a key player in microglia-mediated neuroinflammation and Alzheimer’s disease. Cell Biosci. 2021;11:78.
Wang X, Zhang S, Lin F, Chu W, Yue S. Elevated galectin-3 levels in the serum of patients With Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2015;30:729–32.
Boza-Serrano A, Ruiz R, Sanchez-Varo R, García-Revilla J, Yang Y, Jimenez-Ferrer I, et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019;138:251–73.
Liu D, Tang H, Li XY, Deng MF, Wei N, Wang X, et al. Targeting the HDAC2/HNF-4A/miR-101b/AMPK pathway rescues tauopathy and dendritic abnormalities in Alzheimer’s disease. Mol Ther. 2017;25:752–64.
Wang DB, Kinoshita C, Kinoshita Y, Sopher BL, Uo T, Lee RJ, et al. Neuronal susceptibility to beta-amyloid toxicity and ischemic injury involves histone deacetylase-2 regulation of endophilin-B1. Brain Pathol. 2019;29:164–75.
Datta M, Staszewski O, Raschi E, Frosch M, Hagemeyer N, Tay TL, et al. Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity. 2018;48:514–29.e6.
Cavanagh C, Wong TP. Preventing synaptic deficits in Alzheimer’s disease by inhibiting tumor necrosis factor alpha signaling. IBRO Rep. 2018;4:18–21.
Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, et al. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–71.
Tataryn NM, Singh V, Dyke JP, Berk-Rauch HE, Clausen DM, Aronowitz E, et al. Vascular endothelial growth factor associated dissimilar cerebrovascular phenotypes in two different mouse models of Alzheimer’s disease. Neurobiol Aging. 2021;107:96–108.
Palladino G, Nicolia V, Kovacs GG, Canterini S, Ciraci V, Fuso A, et al. Sexually dimorphic expression of reelin in the brain of a mouse model of Alzheimer disease. J Mol Neurosci. 2017;61:359–67.
Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–70.
López-Gambero AJ, Rosell-Valle C, Medina-Vera D, Navarro JA, Vargas A, Rivera P, et al. A negative energy balance is associated with metabolic dysfunctions in the hypothalamus of a humanized preclinical model of Alzheimer’s disease, the 5×FAD mouse. Int J Mol Sci. 2021;22:5365.
Kameno K, Hasegawa Y, Hayashi K, Takemoto Y, Uchikawa H, Mukasa A, et al. Loss of body weight in old 5×FAD mice and the alteration of gut microbiota composition. Exp Gerontol. 2022;166:111885.
Pádua MS, Guil-Guerrero JL, Lopes PA. Behaviour hallmarks in Alzheimer’s disease 5×FAD mouse model. Int J Mol Sci. 2024;25:6766.
Kanno T, Tsuchiya A, Nishizaki T. Hyperphosphorylation of Tau at Ser396 occurs in the much earlier stage than appearance of learning and memory disorders in 5×FAD mice. Behav Brain Res. 2014;274:302–6.
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–40.
Fernandes A, Caldeira C, Cunha C, Ferreiro E, Vaz AR, Brites D. Differences in immune-related genes underlie temporal and regional pathological progression in 3×Tg-AD mice. Cells. 2022;11:137.
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21.
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–70.
Stimmell AC, Baglietto-Vargas D, Moseley SC, Lapointe V, Thompson LM, LaFerla FM, et al. Impaired spatial reorientation in the 3×Tg-AD mouse model of Alzheimer’s disease. Sci Rep. 2019;9:1311.
Caruso D, Barron AM, Brown MA, Abbiati F, Carrero P, Pike CJ, et al. Age-related changes in neuroactive steroid levels in 3×Tg-AD mice. Neurobiol Aging. 2013;34:1080–9.
Dennison JL, Ricciardi NR, Lohse I, Volmar CH, Wahlestedt C. Sexual dimorphism in the 3×Tg-AD mouse model and its impact on pre-clinical research. J Alzheimers Dis. 2021;80:41–52.
Yang JT, Wang ZJ, Cai HY, Yuan L, Hu MM, Wu MN, et al. Sex differences in neuropathology and cognitive behavior in APP/PS1/tau triple-transgenic mouse model of Alzheimer’s disease. Neurosci Bull. 2018;34:736–46.
Roda AR, Serra-Mir G, Montoliu-Gaya L, Tiessler L, Villegas S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen Res. 2022;17:1666–74.
Gu L, Guo Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J Neurochem. 2013;126:305–11.
Kwak SS, Washicosky KJ, Brand E, von Maydell D, Aronson J, Kim S, et al. Amyloid-β42/40 ratio drives tau pathology in 3D human neural cell culture models of Alzheimer’s disease. Nat Commun. 2020;11:1377.
Qu C, Li QP, Su ZR, Ip SP, Yuan QJ, Xie YL, et al. Nano-Honokiol ameliorates the cognitive deficits in TgCRND8 mice of Alzheimer’s disease via inhibiting neuropathology and modulating gut microbiota. J Adv Res. 2021;35:231–43.
Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–39.
Stathas S, Alvarez VE, Xia W, Nicks R, Meng G, Daley S, et al. Tau phosphorylation sites serine202 and serine396 are differently altered in chronic traumatic encephalopathy and Alzheimer’s disease. Alzheimers Dement. 2022;18:1511–22.
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018;4:575–90.
Davidson R, Krider RI, Borsellino P, Noorda K, Alhwayek G, Vida TA. Untangling tau: molecular insights into neuroinflammation, pathophysiology, and emerging immunotherapies. Curr Issues Mol Biol. 2023;45:8816–39.
Avila-Muñoz E, Arias C. When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res Rev. 2014;18:29–40.
Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187:6539–49.
Kitazawa M, Trinh DN, LaFerla FM. Inflammation induces tau pathology in inclusion body myositis model via glycogen synthase kinase-3beta. Ann Neurol. 2008;64:15–24.
Gao J, Liu J, Li Y, Liu J, Wang H, Chai M, et al. Targeting p53 for neuroinflammation: new therapeutic strategies in ischemic stroke. J Neurosci Res. 2023;101:1393–408.
Prins CA, Almeida FM, Martinez AM. Absence of galectin-3 attenuates neuroinflammation improving functional recovery after spinal cord injury. Neural Regen Res. 2016;11:92–3.
Stajic D, Selakovic D, Jovicic N, Joksimovic J, Arsenijevic N, Lukic ML, et al. The role of galectin-3 in modulation of anxiety state level in mice. Brain Behav Immun. 2019;78:177–87.
Jiao FZ, Wang Y, Zhang HY, Zhang WB, Wang LW, Gong ZJ. Histone deacetylase 2 inhibitor CAY10683 alleviates lipopolysaccharide induced neuroinflammation through attenuating TLR4/NF-κB signaling pathway. Neurochem Res. 2018;43:1161–70.
Wang X, Xue Y, Yao Y, Li Y, Ji X, Chi T, et al. PINK1 regulates mitochondrial fission/fusion and neuroinflammation in β-amyloid-induced Alzheimer’s disease models. Neurochem Int. 2022;154:105298.
Sun XY, Zheng T, Yang X, Liu L, Gao SS, Xu HB, et al. HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J Neuroinflammation. 2019;16:249.
Wu Z, Wang ZH, Liu X, Zhang Z, Gu X, Yu SP, et al. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog Neurobiol. 2020;185:101730.
Wang Y, Dykes GA. Direct modulation of the gut microbiota as a therapeutic approach for Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2022;21:14–25.
Szablewski L. Human gut microbiota in health and Alzheimer’s disease. J Alzheimers Dis. 2018;62:549–60.
O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350:1214–5.
Wang SS, Li XH, Liu P, Li J, Liu L. The relationship between Alzheimer’s disease and intestinal microflora structure and inflammatory factors. Front Aging Neurosci. 2022;14:972982.
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537.
Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802.
Guo X, Li J, Tang R, Zhang G, Zeng H, Wood RJ, et al. High fat diet alters gut microbiota and the expression of paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediators Inflamm. 2017;2017:9474896.
Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:98.
Killingsworth J, Sawmiller D, Shytle RD. Propionate and Alzheimer’s disease. Front Aging Neurosci. 2021;12:580001.
Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90.
Yun Y, Kim HN, Kim SE, Heo SG, Chang Y, Ryu S, et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017;17:151.
Sun ZZ, Li XY, Wang S, Shen L, Ji HF. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl Microbiol Biotechnol. 2020;104:3507–15.
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63:1337–46.
Zhao W, Wang J, Latta M, Wang C, Liu Y, Ma W, et al. Rhizoma gastrodiae water extract modulates the gut microbiota and pathological changes of P-TauThr231 to protect against cognitive impairment in mice. Front Pharmacol. 2022;13:903659.
Wang J, Zhu X, Li Y, Guo W, Li M. Jiedu-Yizhi formula alleviates neuroinflammation in AD rats by modulating the gut microbiota. Evid Based Complement Altern Med. 2022;2022:4023006.
Zhou H, Tai J, Xu H, Lu X, Meng D. Xanthoceraside could ameliorate Alzheimer’s disease symptoms of rats by affecting the gut microbiota composition and modulating the endogenous metabolite levels. Front Pharmacol. 2019;10:1035.
Zhang Z, Tan X, Sun X, Wei J, Li QX, Wu Z. Isoorientin affects markers of Alzheimer’s disease via effects on the oral and gut microbiota in APP/PS1 mice. J Nutr. 2022;152:140–52.
Tsering J, Chen Q, Li H, Han Y, Wu J, Yin H, et al. Effects of the Tibetan medicine Byur dMar Nyer lNga Ril Bu on Alzheimer’s disease in mice models. J Ethnopharmacol. 2022;283:114724.
Li J, Liao X, Yin X, Deng Z, Hu G, Zhang W, et al. Gut microbiome and serum metabolome profiles of capsaicin with cognitive benefits in APP/PS1 mice. Nutrients. 2022;15:118.
Zhang J, Hao J, Liu R, Wu T, Liu R, Sui W, et al. Hawthorn flavonoid ameliorates cognitive deficit in mice with Alzheimer’s disease by increasing the levels of Bifidobacteriales in gut microbiota and docosapentaenoic acid in serum metabolites. Food Funct. 2022;13:12371–82.
Sun P, Zhu H, Li X, Shi W, Guo Y, Du X, et al. Comparative metagenomics and metabolomes reveals abnormal metabolism activity is associated with gut microbiota in Alzheimer’s disease mice. Int J Mol Sci. 2022;23:11560.
Cao J, Amakye WK, Qi C, Liu X, Ma J, Ren J. Bifidobacterium lactis probio-M8 regulates gut microbiota to alleviate Alzheimer’s disease in the APP/PS1 mouse model. Eur J Nutr. 2021;60:3757–69.
Megur A, Baltriukienė D, Bukelskienė V, Burokas A. The microbiota-gut-brain axis and Alzheimer’s disease: neuroinflammation is to blame? Nutrients. 2020;13:37.
Ramis IB, Vianna JS, Gonçalves CV, von Groll A, Dellagostin OA, da Silva PEA. Polymorphisms of the IL-6, IL-8 and IL-10 genes and the risk of gastric pathology in patients infected with Helicobacter pylori. J Microbiol Immunol Infect. 2017;50:153–9.
Li C, Wang N, Zheng G, Yang L. Oral administration of resveratrol-selenium-peptide nanocomposites alleviates Alzheimer’s disease-like pathogenesis by inhibiting Aβ aggregation and regulating gut microbiota. ACS Appl Mater Interfaces. 2021;13:46406–20.
Yang L, Wang Y, Li Z, Wu X, Mei J, Zheng G. Brain targeted peptide-functionalized chitosan nanoparticles for resveratrol delivery: impact on insulin resistance and gut microbiota in obesity-related Alzheimer’s disease. Carbohydr Polym. 2023;310:120714.
Funding
This work was supported by the National Natural Science Foundation of China (Project No. 82104414), Guangdong Basic and Applied Basic Research Foundation (Project No. 2019A1515011257 and 2022A1515011682), and a Direct Grant from the Faculty of Medicine, The Chinese University of Hong Kong (Project No. 2021.071).
Author information
Authors and Affiliations
Contributions
YFX and ZXL conceived the research idea and designed the experimental protocols. MZ performed the experiments and collected the experimental data. RTZ, XQH, QQX, and WY helped with the data analysis. MZ drafted the manuscript. MQH, ZXL, and YFX revised the manuscript. All authors read and approved the final manuscript. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental procedures were approved by the Animal Experimentation Ethics Committee of CUHK (Ref. No.: 21/067/NSF).
Consent for publication
All authors have consented for publication.
Additional information
Consent for publication All authors have consented for publication.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, M., Xu, Qq., Huang, Mq. et al. Rhynchophylline alleviates cognitive deficits in multiple transgenic mouse models of Alzheimer’s disease via modulating neuropathology and gut microbiota. Acta Pharmacol Sin 46, 1813–1833 (2025). https://doi.org/10.1038/s41401-025-01475-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01475-0