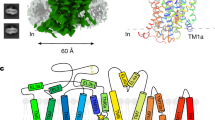
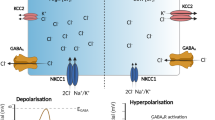
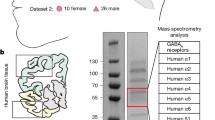
Abstract
Cullin 4B (CUL4B) is the scaffold protein in the CUL4B-RING E3 ubiquitin ligase (CRL4B) complex. Loss-of-function mutations in the human CUL4B gene result in syndromic X-linked intellectual disability (XLID). In addition to intellectual disability, patients with CUL4B mutations exhibit epilepsy. To date, the mechanism underlying epilepsy associated with CUL4B mutation has not been elucidated. Here, we show that male mice with Cul4b deleted in the nervous system are more susceptible to both pentylenetetrazole (PTZ)- and kainic acid (KA)-induced epilepsy and exhibit spontaneous epilepsy without any chemical inducers. We identify the CRL4B complex as an E3 ubiquitin ligase that targets GABA transporter 1 (GAT1). CUL4B deletion in male mice results in GAT1 accumulation and increased GABA reuptake, leading to impaired GABA-mediated inhibitory synaptic transmission. Treating CUL4B-deficient mice with the GAT1 inhibitor tiagabine effectively reverses the increased susceptibility to chemical-induced epilepsy and attenuates spontaneous epilepsy without the use of chemical inducers. We further confirm the role of CUL4B in the regulation of GAT1 levels and GABA uptake in neurons and astrocytes differentiated from induced pluripotent stem cells (iPSCs) derived from patients with CUL4B loss-of-function mutations. Our work reveals a novel mechanism underlying the pathogenesis of epilepsy and identifies a promising drug target for treating CUL4B mutation-associated epilepsy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the raw data supporting the findings of this study are available from the corresponding authors upon request.
References
Tarpey PS, Raymond FL, O’Meara S, Edkins S, Teague J, Butler A, et al. Mutations in CUL4B, which encodes a ubiquitin E3 ligase subunit, cause an X-linked mental retardation syndrome associated with aggressive outbursts, seizures, relative macrocephaly, central obesity, hypogonadism, pes cavus, and tremor. Am J Hum Genet. 2007;80:345–52.
Zou Y, Liu Q, Chen B, Zhang X, Guo C, Zhou H, et al. Mutation in CUL4B, which encodes a member of cullin-RING ubiquitin ligase complex, causes X-linked mental retardation. Am J Hum Genet. 2007;80:561–6.
Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–43.
Chen CY, Tsai MS, Lin CY, Yu IS, Chen YT, Lin SR, et al. Rescue of the genetically engineered Cul4b mutant mouse as a potential model for human X-linked mental retardation. Hum Mol Genet. 2012;21:4270–85.
Jackson S, Xiong Y. CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci. 2009;34:562–70.
Liu L, Yin Y, Li Y, Prevedel L, Lacy EH, Ma L, et al. Essential role of the CUL4B ubiquitin ligase in extra-embryonic tissue development during mouse embryogenesis. Cell Res. 2012;22:1258–69.
Jiang B, Zhao W, Yuan J, Qian Y, Sun W, Zou Y, et al. Lack of Cul4b, an E3 ubiquitin ligase component, leads to embryonic lethality and abnormal placental development. PLoS One. 2012;7:e37070.
Lin CY, Chen CY, Yu CH, Yu IS, Lin SR, Wu JT, et al. Human X-linked intellectual disability factor CUL4B is required for post-meiotic sperm development and male fertility. Sci Rep. 2016;6:20227.
Yin Y, Liu L, Yang C, Lin C, Veith GM, Wang C, et al. Cell autonomous and nonautonomous function of CUL4B in mouse spermatogenesis. J Biol Chem. 2016;291:6923–35.
Li P, Song Y, Zan W, Qin L, Han S, Jiang B, et al. Lack of CUL4B in adipocytes promotes PPARgamma-mediated adipose tissue expansion and insulin sensitivity. Diabetes. 2017;66:300–13.
Hu H, Yang Y, Ji Q, Zhao W, Jiang B, Liu R, et al. CRL4B catalyzes H2AK119 monoubiquitination and coordinates with PRC2 to promote tumorigenesis. Cancer Cell. 2012;22:781–95.
Qian Y, Yuan J, Hu H, Yang Q, Li J, Zhang S, et al. The CUL4B/AKT/beta-catenin axis restricts the accumulation of myeloid-derived suppressor cells to prohibit the establishment of a tumor-permissive microenvironment. Cancer Res. 2015;75:5070–83.
Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–73.
Ghit A, Assal D, Al-Shami AS, Hussein DEE. GABA(A) receptors: structure, function, pharmacology, and related disorders. J Genet Eng Biotechnol. 2021;19:123.
Evenseth LSM, Gabrielsen M, Sylte I. The GABA(B) receptor-structure, ligand binding and drug development. Molecules. 2020;25:3093.
Tang X, Jaenisch R, Sur M. The role of GABAergic signalling in neurodevelopmental disorders. Nat Rev Neurosci. 2021;22:290–307.
Koh W, Kwak H, Cheong E, Lee CJ. GABA tone regulation and its cognitive functions in the brain. Nat Rev Neurosci. 2023;24:523–39.
Wang Y, Wang Y, Chen Z. Double-edged GABAergic synaptic transmission in seizures: the importance of chloride plasticity. Brain Res. 2018;1701:126–36.
Mermer F, Poliquin S, Rigsby K, Rastogi A, Shen W, Romero-Morales A, et al. Common molecular mechanisms of SLC6A1 variant-mediated neurodevelopmental disorders in astrocytes and neurons. Brain. 2021;144:2499–512.
Lyu S, Guo Y, Zhang L, Wang Y, Tang G, Li R, et al. Blockade of GABA transporter-1 and GABA transporter-3 in the lateral habenula improves depressive-like behaviors in a rat model of Parkinson’s disease. Neuropharmacology. 2020;181:108369.
Yang X, Hou D, Jiang W, Zhang C. Intercellular protein-protein interactions at synapses. Protein Cell. 2014;5:420–44.
Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, et al. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–6.
Fattorini G, Melone M, Conti F. A reappraisal of GAT-1 localization in neocortex. Front Cell Neurosci. 2020;14:9.
Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther. 2010;125:394–401.
Motiwala Z, Aduri NG, Shaye H, Han GW, Lam JH, Katritch V, et al. Structural basis of GABA reuptake inhibition. Nature. 2022;606:820–6.
Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: a comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44.
Wang HT, Zhu ZA, Li YY, Lou SS, Yang G, Feng X, et al. CDKL5 deficiency in forebrain glutamatergic neurons results in recurrent spontaneous seizures. Epilepsia. 2021;62:517–28.
Yang Q, Zheng F, Hu Y, Yang Y, Li Y, Chen G, et al. ZDHHC8 critically regulates seizure susceptibility in epilepsy. Cell Death Dis. 2018;9:795.
Jiang CH, Wei M, Zhang C, Shi YS. The amino-terminal domain of GluA1 mediates LTP maintenance via interaction with neuroplastin-65. Proc Natl Acad Sci USA. 2021;118:e2019194118.
Li Y, Zhu K, Li N, Wang X, Xiao X, Li L, et al. Reversible GABAergic dysfunction involved in hippocampal hyperactivity predicts early-stage Alzheimer disease in a mouse model. Alzheimers Res Ther. 2021;13:114.
Egawa K, Kitagawa K, Inoue K, Takayama M, Takayama C, Saitoh S, et al. Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of Angelman syndrome. Sci Transl Med. 2012;4:163ra157.
Jiang W, Wei M, Liu M, Pan Y, Cao D, Yang X, et al. Identification of protein tyrosine phosphatase receptor type O (PTPRO) as a synaptic adhesion molecule that promotes synapse formation. J Neurosci. 2017;37:9828–43.
Ma Y, Hu JH, Zhao WJ, Fei J, Yu Y, Zhou XG, et al. Overexpression of gamma-aminobutyric acid transporter subtype I leads to susceptibility to kainic acid-induced seizure in transgenic mice. Cell Res. 2001;11:61–7.
Liu X, Yang X, Li Y, Wang X, Ma J, Jiang W, et al. Generation of patient-specific pluripotent induced stem cell line SDUBMSI002-A from a patient with X-linked mental retardation syndrome. Stem Cell Res. 2020;43:101724.
Guan J, Liu X, Zhang H, Lv Y, Wang X, Yang X, et al. Generation of an iPSC line (SDQLCHi015-A) from peripheral blood mononuclear cells of a patient with mental retardation type 15 carrying c.1007_1011del, p.(Ile336fs) in CUL4B gene. Stem Cell Res. 2019;41:101628.
Ma Y, Liu X, Zhou M, Sun W, Jiang B, Liu Q, et al. CUL4B mutations impair human cortical neurogenesis through PP2A-dependent inhibition of AKT and ERK. Cell Death Dis. 2024;15:121.
Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8:1670–9.
Zhao W, Jiang B, Hu H, Zhang S, Lv S, Yuan J, et al. Lack of CUL4B leads to increased abundance of GFAP-positive cells that is mediated by PTGDS in mouse brain. Hum Mol Genet. 2015;24:4686–97.
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–6.
Della Vecchia S, Lopergolo D, Trovato R, Pasquariello R, Ferrari AR, Bartolini E. CUL4B-associated epilepsy: report of a novel truncating variant promoting drug-resistant seizures and systematic review of the literature. Seizure. 2023;104:32–37.
Zhao WJ, Ma YH, Fei J, Mei ZT, Guo LH. Increase in drug-induced seizure susceptibility of transgenic mice overexpressing GABA transporter-1. Acta Pharmacol Sin. 2003;24:991–5.
Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003;90:1363–74.
Wu Y, Wang W, Richerson GB. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci. 2001;21:2630–9.
Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–65.
Savtchenko L, Megalogeni M, Rusakov DA, Walker MC, Pavlov I. Synaptic GABA release prevents GABA transporter type-1 reversal during excessive network activity. Nat Commun. 2015;6:6597.
Bhatt M, Gauthier-Manuel L, Lazzarin E, Zerlotti R, Ziegler C, Bazzone A, et al. A comparative review on the well-studied GAT1 and the understudied BGT-1 in the brain. Front Physiol. 2023;14:1145973.
Acknowledgements
We thank the Translational Medicine Core Facility of Shandong University and the School of Basic Medical Sciences Core Facility of Shandong University for consultation and instrument availability. This work was supported by grants from the National Key R&D Program of China (2022YFC2703700 [2022YFC2703701] to YQG and 2022YFC2703700 [2022YFC2703703] to GPS) and the National Natural Science Foundation of China (32370652 and 82171851 to YQG; 31970559 to BCJ).
Author information
Authors and Affiliations
Contributions
YQG, BCJ, and GPS conceived the study concept and design. WJ performed most of the experiments. YYM helped establish the iPSCs. YFW, SQJ, RQY, SXC, and YFG helped establish the experiments. CZ, YZ, MLW, YXZ, QL, and YS participated in the acquisition, analysis, and interpretation of the data. YQG, BCJ, and GPS wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, W., Ma, Yy., Wang, Yf. et al. GABA transporter 1 is a promising drug target for CUL4B mutation-associated epilepsy. Acta Pharmacol Sin 46, 1580–1591 (2025). https://doi.org/10.1038/s41401-025-01490-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01490-1