Abstract
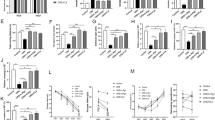
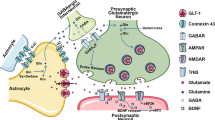
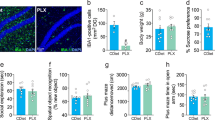
Although significant progress has been made in the development of antidepressants, a large subpopulation of individuals remains unresponsive to existing treatments. Ginsenoside Rg1 (Rg1), a natural compound with well-defined antidepressant effects and low-cost administrations, holds therapeutic promise but requires mechanistic elucidation for clinical translation. Based on our previous finding that Rg1 rescued astrocytic connexin43 (Cx43) downregulation in depression models, we investigated its brain-wide effects and molecular mechanisms in chronic unpredictable stress (CUS)-induced rats. Male rats subjected to CUS received Rg1 (40 mg· kg−1 ·d−1, i.g.) for 8 weeks. Multimodal neuroimaging (fMRI and PET/CT) revealed that Rg1 restored functional connectivity and ameliorated neuroinflammation in CUS rats, with the prelimbic area identified as a critical target region. Through integrated proteomic profiling, molecular docking, and surface plasmon resonance analyses, we pinpointed Cx43-mediated gap junction as the primary target underlying Rg1’s therapeutic action. Mechanistically, we showed that Yes-associated protein (YAP), the primary effector of the Hippo pathway, was translocated into the nucleus to promote the expression of specific genes including those involved in inflammation. Notably, we demonstrated that Rg1 potentiated the Cx43-YAP interaction in the cytoplasm and restricted YAP nuclear translocation activity. The degradation of Cx43 and potentiation of YAP nuclear translocation might represent a novel mechanism for the pathogenesis of depression. Specific blockade of Cx43-based gap junctions, knockdown of Cx43 expression in primary cultured astrocytes, and conditional knockout of astrocytic Cx43 in mice promoted YAP nuclear translocation and retarded the antidepressant effects of Rg1. Accordingly, the Cx43-YAP connection may represent a potential therapeutic target for the antidepressant mechanism of Rg1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Marx W, Penninx BWJH, Solmi M, Furukawa TA, Firth J, Carvalho AF, et al. Major depressive disorder. Nat Rev Dis Prim. 2023;9:44
Stecher C, Cloonan S, Domino ME. The economics of treatment for depression. Annu Rev Public Heal. 2024;45:527–51.
Wang HQ, Wang ZZ, Chen NH. The receptor hypothesis and the pathogenesis of depression: genetic bases and biological correlates. Pharmacol Res. 2021;167:105542
Kishi T, Ikuta T, Sakuma K, Okuya M, Hatano M, Matsuda Y, et al. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: a systematic review and network meta-analysis. Mol Psychiatry. 2023;28:402–9.
Lewis G, Lewis G. Discontinuation symptoms of antidepressants. Lancet Psychiatry. 2024;11:485–6.
Yang IH, Chen YS, Li JJ, Liang YJ, Lin TC, Jakfar S, et al. The development of laminin-alginate microspheres encapsulated with ginsenoside Rg1 and ADSCs for breast reconstruction after lumpectomy. Bioact Mater. 2021;6:1699–710.
Liu Q, Zhang FG, Zhang WS, Pan A, Yang YL, Liu JF, et al. Ginsenoside Rg1 inhibits glucagon-induced hepatic gluconeogenesis through Akt-FoxO1 interaction. Theranostics. 2017;7:4001–12.
Lynch CJ, Elbau IG, Ng T, Ayaz A, Zhu S, Wolk D, et al. Frontostriatal salience network expansion in individuals in depression. Nature. 2024;633:624–33.
Gordon EM, Chauvin RJ, Van AN, Rajesh A, Nielsen A, Newbold DJ, et al. A somato-cognitive action network alternates with effector regions in motor cortex. Nature. 2023;617:351–9.
Herzog S, Bartlett EA, Zanderigo F, Galfalvy HC, Burke A, Mintz A, et al. Neuroinflammation, stress-related suicidal ideation, and negative mood in depression. JAMA Psychiatry. 2024;82:e243543.
Li X, Ramos-Rolón AP, Kass G, Pereira-Rufino LS, Shifman N, Shi Z, et al. Imaging neuroinflammation in individuals with substance use disorders. J Clin Invest. 2024;134:e172884.
Lou YX, Wang ZZ, Xia CY, Mou Z, Ren Q, Liu DD, et al. The protective effect of ginsenoside Rg1 on depression may benefit from the gap junction function in hippocampal astrocytes. Eur J Pharmacol. 2020;882:173309
Simard M, Couldwell WT, Zhang W, Song H, Liu S, Cotrina ML, et al. Glucocorticoids - potent modulators of astrocytic calcium signaling. Glia. 1999;28:1–12.
Levin M. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell. 2021;184:1971–89.
Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99.
Wang HQ, Yang XY, Lai HQ, Sun Y, Yan X, Ai Q, et al. Novel antidepressant mechanism of hypericin: role of connexin 43-based gap junctions. Biomed Pharmacother. 2023;167:115545
Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–20.
Slavi N, Toychiev AH, Kosmidis S, Ackert J, Bloomfield SA, Wulff H, et al. Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy. Proc Natl Acad Sci USA. 2018;115:E5934–43.
Fumagalli A, Heuninck J, Pizzoccaro A, Moutin E, Koenen J, Séveno M, et al. The atypical chemokine receptor 3 interacts with Connexin 43 inhibiting astrocytic gap junctional intercellular communication. Nat Commun. 2020;11:4855
Fan W, Jurado‐Arjona J, Alanis‐Lobato G, Péron S, Berger C, Andrade‐Navarro MA, et al. The transcriptional co‐activator Yap1 promotes adult hippocampal neural stem cell activation. EMBO J. 2023;42:1–14.
Fang L, Teng H, Wang Y, Liao G, Weng L, Li Y, et al. SET1A-mediated mono-methylation at K342 regulates YAP activation by blocking its nuclear export and promotes tumorigenesis. Cancer Cell. 2018;34:103–18.e9.
Kowalczyk W, Romanelli L, Atkins M, Hillen H, González-Blas CB, Jacobs J, et al. Hippo signaling instructs ectopic but not normal organ growth. Science. 2022;378:eabg3679
Moya IM, Halder G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20:211–26.
Wang HQ, Yang SW, Gao Y, Liu YJ, Li X, Ai QD, et al. Novel antidepressant mechanism of ginsenoside Rg1: regulating biosynthesis and degradation of connexin43. J Ethnopharmacol. 2021;278:114212
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–22.
MacAskill MG, Stadulyte A, Williams L, Morgan TEF, Sloan NL, Alcaide-Corral CJ, et al. Quantification of macrophage-driven inflammation during myocardial infarction with 18F-LW223, a novel TSPO radiotracer with binding independent of the rs6971 human polymorphism. J Nucl Med. 2021;62:536–44.
Tan Z, Haider A, Zhang S, Chen J, Wei J, Liao K, et al. Quantitative assessment of translocator protein (TSPO) in the non-human primate brain and clinical translation of [18F]LW223 as a TSPO-targeted PET radioligand. Pharmacol Res. 2023;189:106681.
Dyring-Andersen B, Løvendorf MB, Coscia F, Santos A, Møller LBP, Colaço AR, et al. Spatially and cell-type resolved quantitative proteomic atlas of healthy human skin. Nat Commun. 2020;11:5587.
Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018;135:529–50.
Xia CY, Wang ZZ, Zhang Z, Chen J, Wang YY, Lou YX, et al. Corticosterone impairs gap junctions in the prefrontal cortical and hippocampal astrocytes via different mechanisms. Neuropharmacology. 2018;131:20–30.
Kong R, Patel AS, Sato T, Jiang F, Yoo S, Bao L, et al. Transcriptional circuitry of NKX2-1 and SOX1 defines an unrecognized lineage subtype of small-cell lung cancer. Am J Respir Crit Care Med. 2022;206:1480–94.
Larga JGB, Munabirul WT, Moin AT. Cutting-edge Bioinformatics strategies for synthesizing Cyclotriazadisulfonamide (CADA) analogs in next-Generation HIV therapies. Sci Rep. 2024;14:29764.
Hoh JH, Lal R, John SA, Revel JP, Arnsdorf MF. Atomic force microscopy and dissection of gap junctions. Science. 1991;253:1405–8.
Yang Y, Ren J, Sun Y, Xue Y, Zhang Z, Gong A, et al. A connexin43/YAP axis regulates astroglial-mesenchymal transition in hemoglobin induced astrocyte activation. Cell Death Differ. 2018;25:1870–84.
Fisher YE, Marquis M, D’Alessandro I, Wilson RI. Dopamine promotes head direction plasticity during orienting movements. Nature. 2022;612:316–22.
Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21:224–44.
Costa RO, Martins LF, Tahiri E, Duarte CB. Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip Rev RNA. 2022;13:e1713.
Dupuis JP, Nicole O, Groc L. NMDA receptor functions in health and disease: old actor, new dimensions. Neuron. 2023;111:2312–28.
Ishtiak-Ahmed K, Musliner KL, Christensen KS, Mortensen EL, Nierenberg AA, Gasse C. Real-world evidence on clinical outcomes of commonly used antidepressants in older adults initiating antidepressants for depression: a nationwide cohort study in denmark. Am J Psychiatry. 2024;181:47–56.
Yang SJ, Wang JJ, Cheng P, Chen LJ, Hu JM, Zhu GQ. Ginsenoside Rg1 in neurological diseases: from bench to bedside. Acta Pharmacol Sin. 2023;44:913–30.
Zhang NN, Jiang H, Wang HQ, Wang YT, Peng Y, Liu YB, et al. Novel antidepressant mechanism of ginsenoside Rg1 in regulating the dysfunction of the glutamatergic system in astrocytes. Int J Mol Sci. 2023;24:1–15.
Zhai K, Duan H, Wang W, Zhao S, Khan GJ, Wang M, et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm Sin B. 2021;11:3493–507.
Lin SS, Zhou B, Chen BJ, Jiang RT, Li B, Illes P, et al. Electroacupuncture prevents astrocyte atrophy to alleviate depression. Cell Death Dis. 2023;14:343.
Meghwani H, Berk BC. MST1 kinase-Cx43-YAP/TAZ pathway mediates disturbed flow endothelial dysfunction. Circ Res. 2022;131:765–7.
Huang HC, Wang TY, Rousseau J, Orlando M, Mungaray M, Michaud C, et al. Biomimetic nanodrug targets inflammation and suppresses YAP/TAZ to ameliorate atherosclerosis. Biomaterials. 2024;306:122505.
Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–15.
Ibar C, Irvine KD. Integration of Hippo-YAP signaling with metabolism. Dev Cell. 2020;54:256–67.
Symons RA, Colella F, Collins FL, Rafipay AJ, Kania K, McClure JJ, et al. Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann Rheum Dis. 2022;81:214–24.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82130109, U2202214), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-020), Hunan University of Chinese Medicine First-Class Discipline Construction Project of Chinese Material Medica (201803).
Author information
Authors and Affiliations
Contributions
NHC and HQW conceived and designed this study. HQW performed experiments, analyzed data, and wrote the manuscript. RFZ, QDA, SWY, XYY, APC, and QY performed experiments and verified data. XY, ZZ, JGX, and SFC provided materials and administrated project. ZZW, YTY, and NHC revised the manuscript and were responsible for funding investigation, data curation, and supervision. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Hq., Zheng, Rf., Ai, Qd. et al. Ginsenoside Rg1 alleviates chronic stress-induced depression in rats by targeting Cx43-YAP axis. Acta Pharmacol Sin 46, 1877–1891 (2025). https://doi.org/10.1038/s41401-025-01515-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01515-9