Abstract
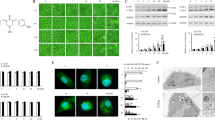
Cancer cells frequently exhibit MHC-I deficiency, impairing immune-mediated cytotoxicity even in the presence of PD-1 checkpoint inhibition. To date, no clinically approved therapies exist that can upregulate MHC-I expression to boost immune responses against cancer cells. Emerging evidence has shown that autophagy plays a role in MHC-I molecule degradation, contributing to reduced recognition of cancer cells by CD8+ T cells. We previously report that fangchinoline, a bisbenzylisoquinoline alkaloid derived from Chinese herb, is a novel autophagy inhibitor with an adjuvant of chemotherapy against lung cancer. In this study we investigated the modulatory effects of PD-1 blockade combined with fangchinoline on CD8+ T cells within the tumor microenvironment of lung cancer. We showed an inverse correlation between elevated autophagic activity and decreased MHC-I surface expression—a phenomenon often associated with poor clinical efficacies—in various human lung cancer cell lines (NCI-H1299, NCI-H1975, A549, NCI-H1650 and NCI-H446) compared with normal bronchial epithelial cells lung cancer. Knockdown of ATG4 and ATG5 resulted in increased MHC-I expression and enhanced tumor antigen presentation in NCI-H1975, NCI-H1299 and A549 cells. As autophagy receptors were crucial for transporting proteins to autophagosomes for degradation, we sequentially silenced various autophagy receptors and found that NDP52 knockdown specifically restored MHC-I expression, suggesting that NDP52-mediated autophagy might contribute to MHC-I degradation, and autophagy inhibition might enhance immune-mediated cancer cell death. We showed that pretreatment of LLC-OVA cells with the autophagy inhibitor fangchinoline (1.25, 2.5, 5 μM) followed by coculture with CD8+ T cells, dose-dependently enhanced immune killing. In both in vitro and in vivo experiments, we showed that fangchinoline combined with anti-PD-1 therapy significantly increased CD8+ T cell–mediated cytotoxicity. In conclusion, this study highlights NDP52 as a key autophagy receptor involved in MHC-I degradation and provides a new insight into tumor immune evasion. Combining autophagy inhibition with immunotherapy may be a promising therapeutic strategy for anticancer immunity enhancement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed in this study are included in the published article. The raw data used for analysis during this study are available from the corresponding author upon reasonable request.
References
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40.
Sun Q, Hong Z, Zhang C, Wang L, Han Z, Ma D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther. 2023;8:320.
Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in lung cancer: current landscape and future directions. Front Immunol. 2022;13:823618.
Qin A, Coffey DG, Warren EH, Ramnath N. Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med. 2016;5:2567–78.
Hurkmans DP, Kuipers ME, Smit J, Van Marion R, Mathijssen RHJ, Postmus PE, et al. Tumor mutational load, CD8+ T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol Immunother. 2020;69:771–7.
Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M. Effect of platinum‑based chemotherapy on the expression of natural killer group 2 member D ligands, programmed cell death‑1 ligand 1 and HLA class I in non‑small cell lung cancer. Oncol Rep. 2019;42:839–48.
Lu ZH, Tu GJ, Fu SL, Shang K, Peng SJ, Chen L, et al. BMI1 induces ubiquitination and protein degradation of Nod-like receptor family CARD domain containing 5 and suppresses human leukocyte antigen class I expression to induce immune escape in non-small cell lung cancer. Kaohsiung J Med Sci. 2022;38:1190–202.
Taylor BC, Balko JM. Mechanisms of MHC-I downregulation and role in immunotherapy response. Front Immunol. 2022;13:844866.
Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol. 2021;12:636568.
White E, Lattime EC, Guo JY. Autophagy regulates stress responses, metabolism, and anticancer immunity. Trends Cancer. 2021;7:778–89.
Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–43.
Rangel M, Kong J, Bhatt V, Khayati K, Guo JY. Autophagy and tumorigenesis. FEBS J. 2022;289:7177–98.
Morishita H, Mizushima N. Diverse cellular roles of autophagy. Annu Rev Cell Dev Biol. 2019;35:453–75.
Mathew R, Khor S, Hackett SR, Rabinowitz JD, Perlman DH, White E. Functional role of autophagy-mediated proteome remodeling in cell survival signaling and innate immunity. Mol Cell. 2014;55:916–30.
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–5.
Zhan L, Zhang J, Zhang J, Liu X, Zhu S, Shi Y, et al. LC3 and NLRC5 interaction inhibits NLRC5-mediated MHC class I antigen presentation pathway in endometrial cancer. Cancer Lett. 2022;529:37–52.
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570.
Gu J, Gui Y, Chen L, Yuan G, Lu HZ, Xu X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS One. 2013;8:e62839.
Yang J, Hu S, Wang C, Song J, Chen C, Fan Y, et al. Fangchinoline derivatives induce cell cycle arrest and apoptosis in human leukemia cell lines via suppression of the PI3K/AKT and MAPK signaling pathway. Eur J Med Chem. 2020;186:111898.
Jung YY, Ha IJ, Um JY, Sethi G, Ahn KS. Fangchinoline diminishes STAT3 activation by stimulating oxidative stress and targeting SHP-1 protein in multiple myeloma model. J Adv Res. 2022;35:245–57.
Ren Z, Song Y, Xian J, Liao Y, Zhan Y, Zhao T, et al. Identification of Fangchinoline as a novel autophagy inhibitor with an adjuvant of chemotherapy against lung cancer. Toxicol Appl Pharmacol. 2023;477:116679.
Jin ZJ. About the evaluation of drug combination. Acta Pharmacol Sin. 2004;25:146–7.
Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560–75.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Sig Transduct Target Ther. 2022;7:196.
Ding J, Wang C, Sun Y, Guo J, Liu S, Cheng Z. Identification of an autophagy-related signature for prognosis and immunotherapy response prediction in ovarian cancer. Biomolecules. 2023;13:339.
Zhu C, Feng X, Tong L, Mu P, Wang F, Quan W, et al. Prediction of acute myeloid leukemia prognosis based on autophagy features and characterization of its immune microenvironment. Front Immunol. 2024;15:1489171.
Gestal‐Mato U, Herhaus L. Autophagy‐dependent regulation of MHC‐I molecule presentation. J Cell Biochem. 2023;125:e30416.
Ye D, Zhou S, Dai X, Xu H, Tang Q, Huang H, et al. Targeting the MHC-I endosomal-lysosomal trafficking pathway in cancer: From mechanism to immunotherapy. Biochim Biophys Acta Rev Cancer. 2024;1879:189161.
Herhaus L, Gestal-Mato U, Eapen VV, Mačinković I, Bailey HJ, Prieto-Garcia C, et al. IRGQ-mediated autophagy in MHC class I quality control promotes tumor immune evasion. Cell. 2024;187:7285–302.e7229.
Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, et al. Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 2018;8:276–87.
Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24:167–85.
Fichtner S, Hose D, Engelhardt M, Meissner T, Neuber B, Krasniqi F, et al. Association of antigen-specific T-cell responses with antigen expression and immunoparalysis in multiple myeloma. Clin Cancer Res. 2015;21:1712–21.
Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124:359–67.
Chang HN, Liu BY, Qi YK, Zhou Y, Chen YP, Pan KM, et al. Blocking of the PD-1/PD-L1 Interaction by a D-peptide antagonist for cancer immunotherapy. Angew Chem. 2015;54:11760–4.
Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27:1492–504.
Gu SS, Zhang W, Wang X, Jiang P, Traugh N, Li Z, et al. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov. 2021;11:1524–41.
Shklovskaya E, Rizos H. MHC class I deficiency in solid tumors and therapeutic strategies to overcome it. Int J Mol Sci. 2021;22:6741.
Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018;9:117.
Memmott RM, Wolfe AR, Carbone DP, Williams TM. Predictors of response, progression-free survival, and overall survival in patients with lung cancer treated with immune checkpoint inhibitors. J Thorac Oncol. 2021;16:1086–98.
Malone T, Schäfer L, Simon N, Heavey S, Cuffe S, Finn S, et al. Current perspectives on targeting PIM kinases to overcome mechanisms of drug resistance and immune evasion in cancer. Pharmacol Ther. 2020;207:107454.
Zhang Q, Zhang Y, Chen Y, Qian J, Zhang X, Yu K. A novel mTORC1/2 Inhibitor (MTI-31) inhibits tumor growth, epithelial-mesenchymal transition, metastases, and improves antitumor immunity in preclinical models of lung cancer. Clin Cancer Res. 2019;25:3630–42.
Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle. 2009;8:4032–9.
Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer 2021;21:298–312.
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–s198.
Cornel AM, Mimpen IL, Nierkens S MHC Class I. Downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers. 2020;12:1760.
Lee JH, Shklovskaya E, Lim SY, Carlino MS, Menzies AM, Stewart A, et al. Transcriptional downregulation of MHC class I and melanoma de-differentiation in resistance to PD-1 inhibition. Nat Commun. 2020;11:1897.
Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–7.
Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020;588:693–8.
Zhang W, Lin L, Zhang Y, Zhao T, Zhan Y, Wang H, et al. Dioscin potentiates the antitumor effect of suicide gene therapy in melanoma by gap junction intercellular communication-mediated antigen cross-presentation. Biomed Pharmacother. 2022;150:112973.
Yao CL, Zhang JQ, Li JY, Wei WL, Wu SF, Guo DA. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Nat Prod Rep. 2021;38:1618–33.
Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm Sin B. 2022;12:1163–85.
Ke M, Zhang Z, Xu B, Zhao S, Ding Y, Wu X, et al. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol. 2019;75:105824.
Cuyas E, Perez-Sanchez A, Micol V, Menendez JA, Bosch-Barrera J. STAT3-targeted treatment with silibinin overcomes the acquired resistance to crizotinib in ALK-rearranged lung cancer. Cell Cycle. 2016;15:3413–8.
Yamamoto K, Venida A, Perera RM, Kimmelman AC. Selective autophagy of MHC-I promotes immune evasion of pancreatic cancer. Autophagy. 2020;16:1524–5.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82104571, 82074070, 82204814), the Natural Science Foundation of Guangdong Province (Grant No. 2022A1515110282), the Science and Technology Plan Project of Guangzhou, China (Grant Nos. 202201011641, 2023A04J2477, 2024A04J10028), the Guangdong Medical Science and Technology Research Foundation (Grant No. A2022488), the Guangdong Provincial Bureau of Traditional Chinese Medicine Research Foundation (Grant Nos. 20222043, 20221115, 20231102), the National Undergraduate Training Programs for Innovation and Entrepreneurship (Grant Nos. 202310572324, 202310572103, 202310572038), and the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (Grant No. pdjh2023b0125).
Author information
Authors and Affiliations
Contributions
Software, Investigation, Methodology, Formal analysis, Visualization, Writing- original draft preparation: YS; Investigation, Methodology, Visualization: YXJ; Methodology, Visualization: FL, JYG; Methodology, Investigation: JBJ, MSX, XYZ; Data curation, Formal analysis: LNH, ZYR, YL, WG; Data curation, Investigation: YJJ; Funding acquisition, Software: SH, XYL; Methodology, Visualization: TXZ, JYG,YFS; Conceptualization, Supervision, Writing-review & editing: HHL; Conceptualization, Formal analysis, Supervision, Writing-review & editing, Funding acquisition: KW; Conceptualization, Funding acquisition, Writing-review & editing: JYX.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of Guangzhou University of Chinese Medicine (Approval No. 20221027002).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., Jiang, Yx., Guan, Jy. et al. Fangchinoline-mediated autophagy inhibition amplifies antigen presentation and PD-1 blockade efficacy in lung cancer. Acta Pharmacol Sin 46, 2751–2764 (2025). https://doi.org/10.1038/s41401-025-01541-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01541-7