Abstract
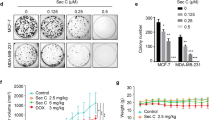
Targeting mitochondrial metabolism represents a novel approach in the discovery and development of anti-tumor drugs. Celastrol (Cel) is a naturally-derived small molecule from Tripterygium wilfordii with significant anticancer activities. In this study, we investigated the anti-tumor mechanisms involving mitochondrial metabolic reprogramming regulated by Cel in breast cancer (BRCA). We showed that Cel potently inhibited the proliferation of triple-negative breast cancer cells (MDA-MB-231) and ER+ breast cancer cells (MCF-7) with IC50 values of 2.15 μM and 2.29 μM, respectively. Administration of Cel (5, 2, 2 mg/kg, i.p. for three times after tumor formation) significantly suppressed the tumor growth in syngeneic allograft and CDX breast cancer mouse models. Using activity-based protein profiling (ABPP) technology, we identified mitochondrial isocitrate dehydrogenases (including IDH2 and IDH3A, collectively referred to as mito-IDHs) as direct targets of Cel. We demonstrated that Cel significantly inhibited mito-IDHs mediated mitochondrial metabolism to induce the accumulation of metabolites α-ketoglutaric acid, and that Cel enhanced the interaction between DPYSL2 with IDH3A while promoting the accumulation of DPYSL2 within mitochondria of BRCA cells resulting in inactivation of JAK/STAT pathway and ultimately induced ferroptosis and apoptosis in cancer cells. Collectively, this study elucidates a pharmacological mechanism by which Cel exerts its tumor-inhibiting effects through modulation of mitochondrial metabolism. Furthermore, it provides compelling evidence supporting Cel as a promising candidate for development as a small-molecule inhibitor targeting mitochondrial metabolism.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res. 2019;150:104511.
Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15.
Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–418.
Momcilovic M, Jones A, Bailey ST, Waldmann CM, Li R, Lee JT, et al. Publisher Correction: In vivo imaging of mitochondrial membrane potential in non-small-cell lung cancer. Nature. 2020;577:E7.
May JL, Kouri FM, Hurley LA, Liu J, Tommasini-Ghelfi S, Ji Y, et al. IDH3alpha regulates one-carbon metabolism in glioblastoma. Sci Adv. 2019;5:eaat0456.
Du B, Sun T, Li X, Diao Y, Li Y. Effect of IDH3a on glucose uptake in lung adenocarcinoma: A pilot study based on [18F]FDG. Cancer Med. 2019;8:5341–51.
Liu X, Qiao Y, Ting X, Si W. Isocitrate dehydrogenase 3A, a rate-limiting enzyme of the TCA cycle, promotes hepatocellular carcinoma migration and invasion through regulation of MTA1, a core component of the NuRD complex. Am J Cancer Res. 2020;10:3212–29.
Li JJ, Yu T, Zeng P, Tian J, Liu P, Qiao S, et al. Wild-type IDH2 is a therapeutic target for triple-negative breast cancer. Nat Commun. 2024;15:3445.
Zeng L, Morinibu A, Kobayashi M, Zhu Y, Wang X, Goto Y, et al. Aberrant IDH3alpha expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene. 2015;34:4758–66.
Lim HY, Ong PS, Wang L, Goel A, Ding L, Li-Ann Wong A, et al. Celastrol in cancer therapy: recent developments, challenges and prospects. Cancer Lett. 2021;521:252–67.
Wang J, Zhang CJ, Chia WN, Loh CC, Li Z, Lee YM, et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat Commun. 2015;6:10111.
Luo P, Liu D, Zhang Q, Yang F, Wong YK, Xia F, et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm Sin B. 2022;12:2300–14.
Zhang Q, Luo P, Xia F, Tang H, Chen J, Zhang J, et al. Capsaicin ameliorates inflammation in a TRPV1-independent mechanism by inhibiting PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem Biol. 2022;29:1248–59.e6.
Huang L, Zhang J, Wei B, Chen S, Zhu S, Qi W, et al. Small-molecule MHC-II inducers promote immune detection and anti-cancer immunity via editing cancer metabolism. Cell Chem Biol. 2023;30:1076–89.e11.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7.
Shin DH, Nam JH, Lee ES, Zhang Y, Kim SJ. Inhibition of Ca2+ release-activated Ca2+ channel (CRAC) by curcumin and caffeic acid phenethyl ester (CAPE) via electrophilic addition to a cysteine residue of Orai1. Biochem Biophys Res Commun. 2012;428:56–61.
Huang L, Wei B, Zhao Y, Gong X, Chen L. DYNLT1 promotes mitochondrial metabolism to fuel breast cancer development by inhibiting ubiquitination degradation of VDAC1. Mol Med. 2023;29:72.
Topf U, Suppanz I, Samluk L, Wrobel L, Boser A, Sakowska P, et al. Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nat Commun. 2018;9:324.
Chasara RS, Ajayi TO, Leshilo DM, Poka MS, Witika BA. Exploring novel strategies to improve anti-tumour efficiency: The potential for targeting reactive oxygen species. Heliyon. 2023;9:e19896.
Lin B, Li Y, Wang T, Qiu Y, Chen Z, Zhao K, et al. CRMP2 is a therapeutic target that suppresses the aggressiveness of breast cancer cells by stabilizing RECK. Oncogene. 2020;39:6024–40.
Abu Rmaileh A, Solaimuthu B, Khatib A, Lavi S, Tanna M, Hayashi A, et al. DPYSL2 interacts with JAK1 to mediate breast cancer cell migration. J Cell Biol. 2022;221:e202106078.
Vasan K, Werner M, Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020;32:341–52.
Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–80.
Zou J, Huang R, Chen Y, Huang X, Li H, Liang P, et al. Dihydropyrimidinase like 2 promotes bladder cancer progression via pyruvate kinase M2-induced aerobic glycolysis and epithelial-mesenchymal transition. Front Cell Dev Biol. 2021;9:641432.
Zu L, He J, Zhou N, Tang Q, Liang M, Xu S. Identification of multiple organ metastasis-associated hub mRNA/miRNA signatures in non-small cell lung cancer. Cell Death Dis. 2023;14:798.
Li F, Ling Q, Lian J, Chen Y, Hu C, Yang M, et al. Dihydropyrimidinase-like 2 can serve as a novel therapeutic target and prognostic biomarker in acute myeloid leukemia. Cancer Med. 2023;12:8319–30.
Soleymani F, Paquet E, Viktor H, Michalowski W, Spinello D. Protein-protein interaction prediction with deep learning: a comprehensive review. Comput Struct Biotechnol J. 2022;20:5316–41.
Meyer LK, Verbist KC, Albeituni S, Scull BP, Bassett RC, Stroh AN, et al. JAK/STAT pathway inhibition sensitizes CD8 T cells to dexamethasone-induced apoptosis in hyperinflammation. Blood. 2020;136:657–68.
Escher TE, Lui AJ, Geanes ES, Walter KR, Tawfik O, Hagan CR, et al. Interaction between MUC1 and STAT1 drives IFITM1 overexpression in aromatase inhibitor-resistant breast cancer cells and mediates estrogen-induced apoptosis. Mol Cancer Res. 2019;17:1180–94.
Wang C, Dai S, Zhao X, Zhang Y, Gong L, Fu K, et al. Celastrol as an emerging anticancer agent: current status, challenges and therapeutic strategies. Biomed Pharmacother. 2023;163:114882.
Yu X, Zhu D, Luo B, Kou W, Cheng Y, Zhu Y. IFNgamma enhances ferroptosis by increasing JAK‑STAT pathway activation to suppress SLCA711 expression in adrenocortical carcinoma. Oncol Rep. 2022;47:97.
Kong R, Wang N, Han W, Bao W, Lu J. IFNgamma-mediated repression of system xc(-) drives vulnerability to induced ferroptosis in hepatocellular carcinoma cells. J Leukoc Biol. 2021;110:301–14.
Liu T, Li Q, Xu X, Li G, Tian C, Zhang T. Molecular mechanisms of anti-cancer bioactivities of seaweed polysaccharides. Chin Herb Med. 2022;14:528–34.
Kong F, Wang C, Zhang J, Wang X, Sun B, Xiao X, et al. Chinese herbal medicines for prostate cancer therapy: From experimental research to clinical practice. Chin Herb Med. 2023;15:485–95.
Acknowledgements
We thank the Artemisinin Research Center, China Academy of Chinese Medical Sciences for assistance with the generation of confocal microscopy data. The schematic diagram was drawn by Figdraw. This work was supported by National Natural Science Foundation of China (82404657, U24A20798); the Natural Science Foundation of Shenzhen for Basic Research (JCYJ20240813104209012) and Guangdong Provincial Enterprise Joint Fund for Basic and Applied Research Foundation (2024A1515220163); Guangdong Basic and Applied Basic Research Foundation (2023A1515110710); the National Key Research and Development Program of China (2020YFA0908000, 2022YFC2303603); the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2023D003, CI2023E005TS05, CI2023E005TS09); the Shenzhen Medical Research Funds (B2302051); the Establishment of Sino-Austria “Belt and Road” Joint Laboratory on Traditional Chinese Medicine for Severe Infectious Diseases and Joint Research (2020YFE0205100); the CACMS Innovation Fund (CI2023E002, CI2021A05101, ZG2024001-05); the Fundamental Research Funds for the Central Public Welfare Research Institutes (ZZ13-ZD-07, ZZ14-YQ-050, ZZ14-FL-010, ZZ14-ND-010, ZZ15-ND-10, ZZ16-ND-10-23, ZZ17-ND-10, ZZ18-ND-10).
Author information
Authors and Affiliations
Contributions
LH contributed to the conception. LH, QLS, CRF, HYL, and PLW designed the project. All authors contributed to data acquisition and analysis. QLS, CRF, HYL, PLW, WHK, and GJL conducted the research, data analysis, figure production, and contributed to draft the manuscript. SJQ performed bioinformatics analysis of the clinical data. PC, XW, RXC, RL, and JZZ performed the mice experiment and LC-MS/MS assay. LH and QLS wrote the original draft of the paper. LH, JGW, PS, and YY provided funding support. All authors reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Ql., Feng, Cr., Li, Hy. et al. Celastrol inhibits the DPYSL2-JAK/STAT pathway by targeting mito-IDHs mediated mitochondrial metabolism to exhaust breast cancer. Acta Pharmacol Sin 46, 2765–2778 (2025). https://doi.org/10.1038/s41401-025-01548-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01548-0