Abstract
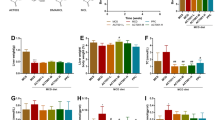
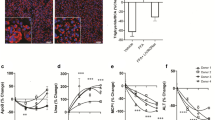
Non-alcoholic steatohepatitis (NASH) has emerged as a prevalent chronic liver disease with a huge unmet clinical need. A few studies have reported the beneficial effects of Poria cocos Wolf (P. cocos) extract on NASH mice, but the active components were still unknown. In this study we investigated the therapeutic effects of dehydrotrametenolic acid methyl ester (ZQS5029-1), a lanosterol-7,9(11)-diene triterpenes in P. cocos, in a high-fat diet plus CCl4 induced murine NASH model and a GAN diet induced ob/ob murine NASH model. The NASH mice were treated with ZQS5029-1 (75 mg·kg–1·d–1, i.g.) for 6 and 8 weeks, respectively. We showed that ZQS5029-1 treatment markedly relieved liver injury, inflammation and fibrosis in both the murine NASH models. We found that ZQS5029-1 treatment significantly suppressed hepatic NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome activation in both the NASH murine models, and blocked lipopolysaccharides (LPS)+adenosine 5’-triphosphate (ATP)/Nigericin-induced NLRP3 inflammasome activation in bone marrow-derived macrophages (BMDMs) and Kupffer cells in vitro. We demonstrated that ZQS5029-1 directly bound to the H236 residue of mouse Caspase-1, thereby inhibiting NLRP3 inflammasome activation. The effects of ZQS5029-1 on macrophage-hepatocyte/HSC crosstalk were analyzed using the supernatants from macrophages preconditioned with LPS + ATP introduced into hepatocytes and hepatic stellate cells (HSCs). We found that the conditioned medium from the BMDMs induced injury and death, as well as lipid accumulation in hepatocytes, and activation of HSCs; these effects were blocked by conditioned medium from BMDMs treated with ZQS5029-1. Moreover, the protective effects of ZQS5029-1 on hepatocytes and HSCs were eliminated by H236A-mutation of Caspase-1. We conclude that ZQS5029-1 is a promising lead compound for the treatment of NASH by inhibiting NLRP3 inflammasome activation through targeting Caspase-1 and regulating the macrophage-hepatocyte/HSC crosstalk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809–17.e28.
Harrison SA, Allen AM, Dubourg J, Noureddin M, Alkhouri N. Challenges and opportunities in NASH drug development. Nat Med. 2023;29:562–73.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–83.
Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37:85–9.
Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22:429–43.
Vande Walle L, Lamkanfi M. Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat Rev Drug Discov. 2024;23:43–66.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20.
Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215:827–40.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606.
Yu L, Hong W, Lu S, Li Y, Guan Y, Weng X, et al. The NLRP3 Inflammasome in non-alcoholic fatty liver disease and steatohepatitis: therapeutic targets and treatment. Front Pharmacol. 2022;13:780496.
Calcagno DM, Chu A, Gaul S, Taghdiri N, Toomu A, Leszczynska A, et al. NOD-like receptor protein 3 activation causes spontaneous inflammation and fibrosis that mimics human NASH. Hepatology. 2022;76:727–41.
Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–44.
Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577–89.
Csak T, Pillai A, Ganz M, Lippai D, Petrasek J, Park JK, et al. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis. Liver Int. 2014;34:1402–13.
Wree A, McGeough MD, Pena CA, Schlattjan M, Li H, Inzaugarat ME, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl). 2014;92:1069–82.
Li Z, Chen Y, Jiang X, Lu P, Wang C, Li Z, et al. Novel sulfonylurea-based NLRP3 inflammasome inhibitor for efficient treatment of nonalcoholic steatohepatitis, endotoxic shock, and colitis. J Med Chem. 2023;66:12966–89.
Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037–46.
Deng YF, Xu QQ, Chen TQ, Ming JX, Wang YF, Mao LN, et al. Kinsenoside alleviates inflammation and fibrosis in experimental NASH mice by suppressing the NF-kappaB/NLRP3 signaling pathway. Phytomedicine. 2022;104:154241.
Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol. 2022;18:461–72.
Cha JY, Kim DH, Chun KH. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Lab Anim Res. 2018;34:133–9.
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12.
Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–6.
Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–53.
Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45–56.
Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–34 e7.
Huang S, Wu Y, Zhao Z, Wu B, Sun K, Wang H, et al. A new mechanism of obeticholic acid on NASH treatment by inhibiting NLRP3 inflammasome activation in macrophage. Metabolism. 2021;120:154797.
Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036–42.
Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in liver fibrosis. Semin Liver Dis. 2017;37:119–27.
Kaufmann B, Kui L, Reca A, Leszczynska A, Kim AD, Booshehri LM, et al. Cell-specific deletion of NLRP3 inflammasome identifies myeloid cells as key drivers of liver inflammation and fibrosis in murine steatohepatitis. Cell Mol Gastroenterol Hepatol. 2022;14:751–67.
Barbier L, Ferhat M, Salame E, Robin A, Herbelin A, Gombert JM, et al. Interleukin-1 family cytokines: keystones in liver inflammatory diseases. Front Immunol. 2019;10:2014.
Atanasov AG, Zotchev SB, Dirsch VM. International natural product sciences T, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–16.
He J, Yang Y, Zhang F, Li Y, Li X, Pu X, et al. Effects of Poria cocos extract on metabolic dysfunction-associated fatty liver disease via the FXR/PPARalpha-SREBPs pathway. Front Pharmacol. 2022;13:1007274.
Kim JH, Sim HA, Jung DY, Lim EY, Kim YT, Kim BJ, et al. Poria cocus Wolf extract ameliorates hepatic steatosis through regulation of lipid metabolism, inhibition of ER stress, and activation of autophagy via AMPK activation. Int J Mol Sci. 2019;20:4801.
Li Y, Wang P, Yang H, He J, Yang Y, Tao Y, et al. In vivo identification of bioactive components of Poria cocos for adjusting mitochondria against metabolic dysfunction-associated fatty liver disease. Heliyon. 2024;10:e35645.
Rios JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011;77:681–91.
Ding X, Li S, Huang H, Shen J, Ding Y, Chen T, et al. Bioactive triterpenoid compounds of Poria cocos (Schw.) Wolf in the treatment of diabetic ulcers via regulating the PI3K-AKT signaling pathway. J Ethnopharmacol. 2024;325:117812.
Gu J-B, Wang J, Yang Y, Ye X-R, Zhang C-X, Zhang X-Y, et al. p-Cymene and pachymic acid alleviate adalimumab-resistance cell line inflammatory response via inhibiting PI3K/AKT/mTOR signaling pathway. Eur J Inflamm. 2024;22:1–10.
Guo ZY, Wu X, Zhang SJ, Yang JH, Miao H, Zhao YY. Poria cocos: traditional uses, triterpenoid components and their renoprotective pharmacology. Acta Pharmacol Sin. 2025;46:836–51.
Hu S, Yang B, Li B, Fan Q, Wu T, Li S, et al. RNA-Seq analysis reveals potential neuroprotective mechanisms of Pachymic Acid toward iron-induced oxidative stress and cell death. Cell Transpl. 2024;33:9636897231218382.
Qin C, Chen X, Hu T, Sun W, Liu Z, Li M, et al. Pachymic Acid alleviates oxidative damage of retinal cells induced by sodium iodate or hydrogen peroxide by activating the Nrf2/HO-1 signaling pathway. Natl Prod Commun. 2024;19:1–10.
Kanematsu A, Natori S. [Triterpenoids of Hoelen (fuling), sclerotia of Poria cocos (Schw.) Wolf. I]. Yakugaku Zasshi. 1970;90:475–9.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Wang HY, Lin X, Huang GG, Zhou R, Lei SY, Ren J, et al. Atranorin inhibits NLRP3 inflammasome activation by targeting ASC and protects NLRP3 inflammasome-driven diseases. Acta Pharmacol Sin. 2023;44:1687–700.
Zhao Z, Deng ZT, Huang S, Ning M, Feng Y, Shen Y, et al. Alisol B alleviates hepatocyte lipid accumulation and lipotoxicity via regulating RARalpha-PPARgamma-CD36 cascade and attenuates non-alcoholic steatohepatitis in mice. Nutrients. 2022;14:2411.
Kubota N, Kado S, Kano M, Masuoka N, Nagata Y, Kobayashi T, et al. A high-fat diet and multiple administration of carbon tetrachloride induces liver injury and pathological features associated with non-alcoholic steatohepatitis in mice. Clin Exp Pharmacol Physiol. 2013;40:422–30.
Hansen HH, Egidius HM, Oro D, Evers SS, Heeboll S, Eriksen PL, et al. Human translatability of the GAN diet-induced obese mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020;20:210.
Boland ML, Oro D, Tolbol KS, Thrane ST, Nielsen JC, Cohen TS, et al. Towards a standard diet-induced and biopsy-confirmed mouse model of non-alcoholic steatohepatitis: Impact of dietary fat source. World J Gastroenterol. 2019;25:4904–20.
Dixon LJ, Berk M, Thapaliya S, Papouchado BG, Feldstein AE. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest. 2012;92:713–23.
Baeza-Raja B, Goodyear A, Liu X, Lam K, Yamamoto L, Li Y, et al. Pharmacological inhibition of P2RX7 ameliorates liver injury by reducing inflammation and fibrosis. PLoS One. 2020;15:e0234038.
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21.
Hoss F, Rodriguez-Alcazar JF, Latz E. Assembly and regulation of ASC specks. Cell Mol Life Sci. 2017;74:1211–29.
Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42.
Inzaugarat ME, Johnson CD, Holtmann TM, McGeough MD, Trautwein C, Papouchado BG, et al. NLR Family Pyrin Domain-Containing 3 inflammasome activation in hepatic stellate cells induces liver fbrosis in mice. Hepatology. 2019;69:845–59.
Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–8.
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–82.
Li L, Zuo ZT, Wang YZ. The Traditional usages, chemical components and pharmacological activities of Wolfiporia cocos: a review. Am J Chin Med. 2022;50:389–440.
Tan YY, Yue SR, Lu AP, Zhang L, Ji G, Liu BC, et al. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-kappaB/CCL3/CCR1 axis. Phytomedicine. 2022;103:154208.
Cheng Y, Xie Y, Ge JC, Wang L, Peng DY, Yu NJ, et al. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr Polym. 2021;263:117979.
Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, et al. NLRP3 inflammasome driven liver injury and fibrosis: roles of IL-17 and TNF in mice. Hepatology. 2018;67:736–49.
Brenner DA, Seki E, Taura K, Kisseleva T, Deminicis S, Iwaisako K, et al. Non-alcoholic steatohepatitis-induced fibrosis: Toll-like receptors, reactive oxygen species and Jun N-terminal kinase. Hepatol Res. 2011;41:683–6.
Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 2021;74:156–67.
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50.
Li H, Zhou Y, Wang H, Zhang M, Qiu P, Zhang M, et al. Crosstalk between liver macrophages and surrounding cells in nonalcoholic steatohepatitis. Front Immunol. 2020;11:1169.
Bai X, Sun SS, Zhou JY. Role of NLRP3 inflammasome in the pathogenesis of alcoholic liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:567–71.
Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–94.e1.
Su T, He Y, Wang M, Zhou H, Huang Y, Ye M, et al. Macrophage-hepatocyte circuits mediated by grancalcin aggravate the progression of metabolic dysfunction associated steatohepatitis. Adv Sci (Weinh). 2024;11:e2406500.
Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–64.
Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, et al. Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci USA. 2009;106:21984–9.
Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100.
Tang Y, Feng B, Wang Y, Sun H, You Y, Yu J, et al. Structure-based discovery of CZL80, a caspase-1 inhibitor with therapeutic potential for febrile seizures and later enhanced epileptogenic susceptibility. Br J Pharmacol. 2020;177:3519–34.
Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–64.
Kazankov K, Jorgensen SMD, Thomsen KL, Moller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59.
Acknowledgements
This study was supported by State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Grant No. SKLDR-2022-LH-08).
Author information
Authors and Affiliations
Contributions
YL, QSZ, and LYX designed the research. LYX, NRY, SLH, HQ, and LQ performed the research. LYX and NRY analyzed and interpreted the data. YL, QSZ, LYX, and NRY wrote the paper. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, Ly., Yu, Nr., Huang, Sl. et al. Dehydrotrametenolic acid methyl ester, a triterpenoid of Poria cocos, alleviates non-alcoholic steatohepatitis by suppressing NLRP3 inflammasome activation via targeting Caspase-1 in mice. Acta Pharmacol Sin 46, 2734–2750 (2025). https://doi.org/10.1038/s41401-025-01569-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01569-9