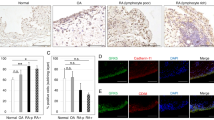
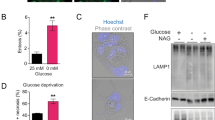
Abstract
Glucose metabolism disorder is an important hallmark of rheumatoid arthritis (RA). Inhibiting key glycolysis enzymes is the primary approach, but effective treatments targeting glycolytic metabolism have not yet reached clinical practice. G protein-coupled receptor kinase 2 (GRK2) as a multi-signals regulatory hub has attracted wide attention. In this study, we investigated the role of GRK2 inhibitor on glycolysis of monocyte-derived macrophages (MDMs), the primary source of inflammatory mediators in RA synovium. Human peripheral mononuclear cells were obtained from RA patients and differentiated into MDMs with M-CSF (100 ng/ml) for 5 days. By analyzing the metabolic status of RA MDMs in normoxia and hypoxia, we found that glycolysis was increased in RA MDMs, and inhibiting glycolysis could suppress the macrophage inflammatory phenotype. The antiglycolytic role of GRK2 deletion was tested in MDMs in vitro and in vivo. We conducted proteomics and mass spectrometric analysis and confirmed the inhibitory role of GRK2 on several key glycolytic enzymes. GRK2 maintained PKM2 tetramer stability through two synergistic modifications—phosphorylation at S406 and de-succinylation at K433. In RA, decreased cytoplasmic GRK2 protein levels impaired its regulation toward PKM2, leading to enhanced glycolysis and accelerating RA progression. Administration of GRK2 inhibitors paroxetine, CP-25, or the glycolysis inhibitor 2-DG for 21 days in the CIA mouse model all restored cytoplasmic GRK2 levels and homeostatic regulation, offering a potential therapeutic approach for RA glycolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Brown P, Pratt AG, Hyrich KL. Therapeutic advances in rheumatoid arthritis. BMJ. 2024;384:e070856.
Yang X, Zhang W, Wang L, Zhao Y, Wei W. Metabolite-sensing GPCRs in rheumatoid arthritis. Trends Pharmacol Sci. 2024;45:118–33.
Souto-Carneiro M, Tóth L, Behnisch R, Urbach K, Klika KD, Carvalho RA, et al. Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann Rheum Dis. 2020;79:499–506.
Wu B, Qiu J, Zhao TV, Wang Y, Maeda T, Goronzy IN, et al. Succinyl-CoA ligase deficiency in oro-inflammatory and tissue-invasive T cells. Cell Metab. 2020;32:967–80.
Meyer A, Zack SR, Nijim W, Burgos A, Patel V, Zanotti B, et al. Metabolic reprogramming by Syntenin-1 directs RA FLS and endothelial cell-mediated inflammation and angiogenesis. Cell Mol Immunol. 2024;21:33–46.
You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH, et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7:eabe0083.
Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, et al. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci USA. 2009;106:6232–7.
Yang X, Zhao Y, Wei Q, Zhu X, Wang L, Zhang W, et al. GRK2 inhibits Flt-1+ macrophage infiltration and its proangiogenic properties in rheumatoid arthritis. Acta Pharm Sin B. 2024;14:241–55.
Grayson PC, Eddy S, Taroni JN, Lightfoot YL, Mariani L, Parikh H, et al. Metabolic pathways and immunometabolism in rare kidney diseases. Ann Rheum Dis. 2018;77:1226–33.
Liu Z, Xu J, Ma Q, Zhang X, Yang Q, Wang L, et al. Glycolysis links reciprocal activation of myeloid cells and endothelial cells in the retinal angiogenic niche. Sci Transl Med. 2020;12:eaay1371.
Jia N, Gao Y, Li M, Liang Y, Li Y, Lin Y, et al. Metabolic reprogramming of proinflammatory macrophages by target delivered roburic acid effectively ameliorates rheumatoid arthritis symptoms. Signal Transduct Target Ther. 2023;8:280.
Van Raemdonck K, Umar S, Palasiewicz K, Volin MV, Elshabrawy HA, Romay B, et al. Interleukin-34 reprograms glycolytic and osteoclastic rheumatoid arthritis macrophages via syndecan 1 and macrophage colony-stimulating factor receptor. Arthritis Rheumatol. 2021;73:2003–14.
Jing C, Castro-Dopico T, Richoz N, Tuong ZK, Ferdinand JR, Lok LSC, et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc Natl Acad Sci USA. 2020;117:15160–71.
Hanlon MM, McGarry T, Marzaioli V, Amaechi S, Song Q, Nagpal S, et al. Rheumatoid arthritis macrophages are primed for inflammation and display bioenergetic and functional alterations. Rheumatology. 2023;62:2611–20.
Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–34.
Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7:11635.
Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. BMJ. 1998;317:1370–3.
Radoux-Mergault A, Oberhauser L, Aureli S, Gervasio FL, Stoeber M. Subcellular location defines GPCR signal transduction. Sci Adv. 2023;9:eadf6059.
Cheng J, Klei LR, Hubel NE, Zhang M, Schairer R, Maurer LM, et al. GRK2 suppresses lymphomagenesis by inhibiting the MALT1 proto-oncoprotein. J Clin Invest. 2020;130:1036–51.
Evron T, Daigle TL, Caron MG. GRK2: multiple roles beyond G protein-coupled receptor desensitization. Trends Pharmacol Sci. 2012;33:154–64.
Tu J, Chen W, Fang Y, Han D, Chen Y, Jiang H, et al. PU.1 promotes development of rheumatoid arthritis via repressing FLT3 in macrophages and fibroblast-like synoviocytes. Ann Rheum Dis. 2023;82:198–211.
Habtemichael EN, Li DT, Camporez JP, Westergaard XO, Sales CI, Liu X, et al. Insulin-stimulated endoproteolytic TUG cleavage links energy expenditure with glucose uptake. Nat Metab. 2021;3:378–93.
Wang S, Zhou L, Ji N, Sun C, Sun L, Sun J, et al. Targeting ACYP1-mediated glycolysis reverses lenvatinib resistance and restricts hepatocellular carcinoma progression. Drug Resist Updat. 2023;69:100976.
Jia H, Li M, Li W, Liu L, Jian Y, Yang Z, et al. A serine/threonine protein kinase encoding gene KERNEL NUMBER PER ROW6 regulates maize grain yield. Nat Commun. 2020;11:988.
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22.
Han C, Jiang L, Wang W, Zuo S, Gu J, Chen L, et al. GRK2 activates TRAF2–NF-κB signaling to promote hyperproliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Acta Pharm Sin B. 2025. https://doi.org/10.1016/j.apsb.2025.02.031.
Yang Y, Ren P, Liu X, Sun X, Zhang C, Du X, et al. PPP1R26 drives hepatocellular carcinoma progression by controlling glycolysis and epithelial-mesenchymal transition. J Exp Clin Cancer Res. 2022;41:101.
Theret M, Gsaier L, Schaffer B, Juban G, Ben Larbi S, Weiss-Gayet M, et al. AMPKα1-LDH pathway regulates muscle stem cell self-renewal by controlling metabolic homeostasis. EMBO J. 2017;36:1946–62.
Xu J, Jiang C, Wang X, Geng M, Peng Y, Guo Y, et al. Upregulated PKM2 in macrophages exacerbates experimental arthritis via STAT1 signaling. J Immunol. 2020;205:181–92.
Li X, Tian BM, Deng DK, Liu F, Zhou H, Kong DQ, et al. LncRNA GACAT2 binds with protein PKM1/2 to regulate cell mitochondrial function and cement genesis in an inflammatory environment. Bone Res. 2022;10:29.
Qi H, Ning X, Yu C, Ji X, Jin Y, McNutt MA, et al. Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis. 2019;10:170.
Yang XZ, Wei W. CP-25, a compound derived from paeoniflorin: research advance on its pharmacological actions and mechanisms in the treatment of inflammation and immune diseases. Acta Pharmacol Sin. 2020;41:1387–94.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82473931, 82430114 and 82204405), the Natural Science Foundation of Anhui Provincial (No. 2408085MH217), the Young Elite Scientists Sponsorship Program of Anhui Association for Science and Technology (No. RCTJ202427), the Research Fund of Anhui Institute of translational medicine (No. 2022zhyx-B04), and the Anhui Province Excellent Research and Innovation Team Project (No. 2024AH010013).
Author information
Authors and Affiliations
Contributions
WW and XZY conceived and designed the experiments. XZY, WKZ, LPW, ZQZ, WZ, and LLL performed the experiments. XZY, WKZ, and LPW analyzed the data. WW, XZY, YC, and YJZ contributed reagents/materials/analysis tools. XZY wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Xz., Zhang, Wk., Zhu, Zq. et al. GRK2-mediated phosphorylation and de-succinylation of PKM2 reduce macrophage glycolysis in rheumatoid arthritis. Acta Pharmacol Sin 46, 2693–2706 (2025). https://doi.org/10.1038/s41401-025-01582-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01582-y