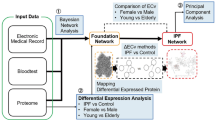
Abstract
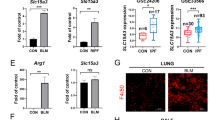
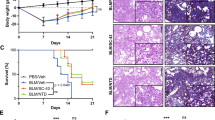
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease; however, effective clinical treatments for IPF are lacking. High serum amyloid A (SAA) expression in serum is closely related to the severity of pulmonary fibrosis, but the underlying mechanisms remain incompletely understood. This study found that the expression of endogenous SAA3 was significantly induced in mice with bleomycin-induced fibrosis. Saa3 deletion alleviated pulmonary fibrosis in mice. Additionally, recombinant IL-36α treatment aggravated fibrosis in bleomycin-induced Saa3−/− mice. Furthermore, SAA3 could induce the expression of IL-36α in macrophages through the NF-κB pathway and transcription factor Krűppel-like factor 6 (KLF6). Also, the Klf6 knockdown alleviated severe lung fibrosis after recombinant SAA3 treatment. In conclusion, our study suggested that SAA3 aggravated bleomycin-induced pulmonary fibrosis by inducing IL-36α expression in macrophages through the NF-κB–KLF6 pathway. It provides new theoretical bases and potential therapeutic targets for treating fibrosis-related diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
References
Somogyi V, Chaudhuri N, Torrisi SE, Kahn N, Müller V, Kreuter M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur Respir Rev. 2019;28:190021.
Glass DS, Grossfeld D, Renna HA, Agarwala P, Spiegler P, DeLeon J, et al. Idiopathic pulmonary fibrosis: current and future treatment. Clin Respir J. 2022;16:84–96.
Torrisi SE, Kahn N, Vancheri C, Kreuter M. Evolution and treatment of idiopathic pulmonary fibrosis. Presse Med. 2020;49:104025.
Yu QY, Tang XX. Irreversibility of pulmonary fibrosis. Aging Dis. 2022;13:73–86.
Ortiz-Zapater E, Signes-Costa J, Montero P, Roger I. Lung fibrosis and fibrosis in the lungs: Is it all about myofibroblasts. Biomedicines. 2022;10:1423.
Ptasinski VA, Stegmayr J, Belvisi MG, Wagner DE, Murray LA. Targeting alveolar repair in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2021;65:347–65.
Moss BJ, Ryter SW, Rosas IO. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu Rev Pathol. 2022;17:515–46.
Spagnolo P, Tonelli R, Samarelli AV, Castelli G, Cocconcelli E, Petrarulo S, et al. The role of immune response in the pathogenesis of idiopathic pulmonary fibrosis: far beyond the Th1/Th2 imbalance. Expert Opin Ther Targets. 2022;26:617–31.
Bocchino M, Zanotta S, Capitelli L, Galati D. Dendritic cells are the intriguing players in the puzzle of idiopathic pulmonary fibrosis pathogenesis. Front Immunol. 2021;12:664109.
Savin IA, Zenkova MA, Sen’kova AV. Pulmonary fibrosis as a result of acute lung inflammation: molecular mechanisms, relevant in vivo models, prognostic and therapeutic approaches. Int J Mol Sci. 2022;23:14959.
Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang CY. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res. 2018;19:170.
Elias M, Zhao S, Le HT, Wang J, Neurath MF, Neufert C, et al. IL-36 in chronic inflammation and fibrosis—bridging the gap? J Clin Invest. 2021;131:e144336.
Neurath MF. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. 2020;55:70–9.
Iznardo H, Puig L. Exploring the role of IL-36 cytokines as a new target in psoriatic disease. Int J Mol Sci. 2021;22:4344.
Wu YR, Hsing CH, Chiu CJ, Huang HY, Hsu YH. Roles of IL-1 and IL-10 family cytokines in the progression of systemic lupus erythematosus: Friends or foes? IUBMB Life. 2022;74:143–56.
Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carlé B, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–97.e11.
Melton E, Qiu H. Interleukin-36 cytokine/receptor signaling: a new target for tissue fibrosis. Int J Mol Sci. 2020;21:6458.
Chi HH, Hua KF, Lin YC, Chu CL, Hsieh CY, Hsu YJ, et al. IL-36 signaling facilitates activation of the NLRP3 inflammasome and IL-23/IL-17 axis in renal inflammation and fibrosis. J Am Soc Nephrol. 2017;28:2022–37.
Zhang Q, Guo L, Song X, Lv C, Tang P, Li Y, et al. Serum IL-36 cytokines levels in idiopathic pulmonary fibrosis and connective tissue disease-associated interstitial lung diseases. Clin Chim Acta. 2022;530:8–12.
Sack GH Jr. Serum amyloid A (SAA) proteins. Subcell Biochem. 2020;94:421–36.
Malle E, Sodin-Semrl S, Kovacevic A. Serum amyloid A: an acute-phase protein involved in tumour pathogenesis. Cell Mol Life Sci. 2009;66:9–26.
Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv Clin Chem. 2019;90:25–80.
Sack GH Jr. Serum amyloid A—a review. Mol Med. 2018;24:46.
Kawasaki H, Murakami T, Badr Y, Kamiya S, Shimizu K, Okada A, et al. In vitro and ex vivo expression of serum amyloid A3 in mouse lung epithelia. Exp Lung Res. 2020;46:352–61.
Bang YJ, Hu Z, Li Y, Gattu S, Ruhn KA, Raj P, et al. Serum amyloid A delivers retinol to intestinal myeloid cells to promote adaptive immunity. Science. 2021;373:eabf9232.
Zhang G, Liu J, Wu L, Fan Y, Sun L, Qian F, et al. Elevated expression of serum amyloid A 3 protects colon epithelium against acute injury through TLR2-dependent induction of neutrophil IL-22 expression in a mouse model of colitis. Front Immunol. 2018;9:1503.
Vercalsteren E, Vranckx C, Vermeire I, Gooijen M, Lijnen R, Scroyen I. Serum amyloid A3 deficiency impairs in vitro and in vivo adipocyte differentiation. Adipocyte. 2021;10:242–50.
Yang X, Li R, Xu L, Qian F, Sun L. Serum amyloid A3 is required for caerulein-induced acute pancreatitis through induction of RIP3-dependent necroptosis. Immunol Cell Biol. 2021;99:34–48.
Djurec M, Graña O, Lee A, Troulé K, Espinet E, Cabras L, et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci USA. 2018;115:E1147–e56.
Vietri L, Bennett D, Cameli P, Bergantini L, Cillis G, Sestini P, et al. Serum amyloid A in patients with idiopathic pulmonary fibrosis. Respir Investig. 2019;57:430–4.
Liang Q, Cai W, Zhao Y, Xu H, Tang H, Chen D, et al. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol Res. 2020;158:104884.
Zhang Y, Lei CQ, Hu YH, Xia T, Li M, Zhong B, et al. Krüppel-like factor 6 is a co-activator of NF-κB that mediates p65-dependent transcription of selected downstream genes. J Biol Chem. 2014;289:12876–85.
Heukels P, Moor CC, von der Thüsen JH, Wijsenbeek MS, Kool M. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med. 2019;147:79–91.
Peng L, Wen L, Shi QF, Gao F, Huang B, Meng J, et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020;11:978.
Bolourani S, Brenner M, Wang P. The interplay of DAMPs, TLR4, and proinflammatory cytokines in pulmonary fibrosis. J Mol Med. 2021;99:1373–84.
Dev S, Singh A. Study of role of serum amyloid A (SAA) as a marker of disease activity in juvenile idiopathic arthritis. J Fam Med Prim Care. 2019;8:2129–33.
Lakota K, Carns M, Podlusky S, Mrak-Poljsak K, Hinchcliff M, Lee J, et al. Serum amyloid A is a marker for pulmonary involvement in systemic sclerosis. PLoS One. 2015;10:e0110820.
Lee JY, Hall JA, Kroehling L, Wu L, Najar T, Nguyen HH, et al. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell. 2020;180:79–91.e16.
Fan Y, Zhang G, Vong CT, Ye RD. Serum amyloid A3 confers protection against acute lung injury in Pseudomonas aeruginosa-infected mice. Am J Physiol Lung Cell Mol Physiol. 2020;318:L314–l22.
Ather JL, Dienz O, Boyson JE, Anathy V, Amiel E, Poynter ME. Serum amyloid A3 is required for normal lung development and survival following influenza infection. Sci Rep. 2018;8:16571.
Vietri L, d’Alessandro M, Bergantini L, Carleo A, Cameli P, Mazzei MA, et al. Specificity of serum amyloid A as a biomarker of idiopathic pulmonary fibrosis. Intern Med J. 2020;50:1571–4.
Zhao D, Abbasi A, Rossiter HB, Su X, Liu H, Pi Y, et al. Serum amyloid A in stable COPD patients is associated with the frequent exacerbator phenotype. Int J Chron Obstruct Pulmon Dis. 2020;15:2379–88.
Ye RD, Sun L. Emerging functions of serum amyloid A in inflammation. J Leukoc Biol. 2015;98:923–9.
He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–9.
Facci L, Barbierato M, Zusso M, Skaper SD, Giusti P. Serum amyloid A primes microglia for ATP-dependent interleukin-1β release. J Neuroinflammation. 2018;15:164.
He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–81.
Bigonnesse F, Marois M, Maheux R, Akoum A. Interleukin-1 receptor accessory protein is constitutively expressed in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 2001;7:333–9.
Murrieta-Coxca JM, Gómez-Chávez F, Baeza-Martínez DA, Cancino-Diaz ME, Cancino-Diaz JC, Pérez-Tapia SM, et al. Estrous cycle and gestational age-dependent expression of members of the interleukin-36 subfamily in a semi-allogeneic model of infected and non-infected murine pregnancy. Front Immunol. 2016;7:376.
Baker JR, Fenwick PS, Koss CK, Owles HB, Elkin SL, Fine J, et al. IL-36 receptor agonist and antagonist imbalance drives neutrophilic inflammation in COPD. JCI Insight. 2022;7:e155581.
Tian S, Zhou X, Zhang M, Cui L, Li B, Liu Y, et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res Ther. 2022;13:330.
Ahronian LG, Zhu LJ, Chen YW, Chu HC, Klimstra DS, Lewis BC. A novel KLF6-Rho GTPase axis regulates hepatocellular carcinoma cell migration and dissemination. Oncogene. 2016;35:4653–62.
Yuce K, Ozkan AI. The kruppel-like factor (KLF) family, diseases, and physiological events. Gene. 2024;895:148027.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2023YFA0913700), National Natural Science Foundation of China (82073858, 82173821, 82273934, 82373875, 82072142 and 82473928), the open project of Anhui Province Key Laboratory of Translational Cancer Research (KFKT202302), Health Profession Clinical Research Funds of the Shanghai Municipal Health Commission (Grant No. 202140315), and the Scientific Research Project funded by the Shanghai Municipal Science and Technology Commission (Grant No. 23ZR1449900). Health Profession Clinical Research Funds of the Shanghai Municipal Health Commission (Grant No. 202140315), and the Scientific Research Project funded by the Shanghai Municipal Science and Technology Commission (Grant No. 23ZR1449900).
Author information
Authors and Affiliations
Contributions
LS and FQ conceived the study. LS and XYY designed, XYY, WL, YL, and QL performed, and interpreted experimental data. XYY, HXL, AJX and YWZ analyzed database. XYY, YHC, TYL and WG prepared the data visualization. LS and XYY wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Xy., Liu, Y., Li, W. et al. Serum amyloid A3 aggravates bleomycin-induced pulmonary fibrosis through Krüppel-like factor 6-dependent interlukin-36α expression. Acta Pharmacol Sin 46, 3244–3256 (2025). https://doi.org/10.1038/s41401-025-01596-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01596-6