Abstract
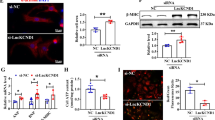
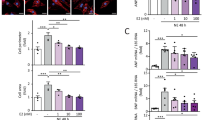
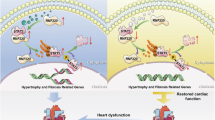
Cardiac hypertrophy as one of the major predisposing factors for chronic heart failure lacks effective interventions. It has been shown that protein ubiquitination plays an important role in cardiac hypertrophy. SMURF2 (SMAD-specific E3 ubiquitin ligase 2) is an important member of NEDD4 (neuronal precursor cell expressed developmentally downregulated 4) family of HECT E3 ubiquitin ligases. In this study we investigated the regulatory role of SMURF2 in cardiac hypertrophy. Experiment models were established in mice by transverse aortic constriction (TAC) in vivo, as well as in neonatal rat cardiomyocytes (NRCMs) by treatment with angiotensin II (Ang II, 1 μM) in vitro. We showed that the expression levels of SMURF2 were significantly elevated in cardiac tissues from patients with cardiac hypertrophy and the two experiment models. In NRCMs, SMURF2 knockdown or treatment with a specific SMURF2 inhibitor heclin (8 μM) significantly inhibited Ang II-induced cardiomyocyte hypertrophy, evidenced by reduced mRNA levels of Anp, Bnp and β-Mhc as well as cell surface. Prophylactic or therapeutic administration of heclin (10 mg·kg–1·d–1, i.p., for 5 or 4 weeks) effectively suppressed TAC-induced cardiac hypertrophy, and rescued heart function. We demonstrated that SMURF2 interacted with AXIN1 and increased ubiquitination degradation of AXIN1 in myocardial tissues, activating the Wnt/β-catenin signaling pathway. Heclin inhibited the ubiquitination degradation of AXIN1 by SMURF2 to alleviate cardiac hypertrophy. In conclusion, upregulated SMURF2 leads to AXIN1 ubiquitination and degradation, thereby facilitating the progression of cardiac hypertrophy. SMURF2 inhibitor heclin may serve as a therapeutic strategy for the treatment of cardiac hypertrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zhao S, Song TY, Wang ZY, Gao J, Cao JW, Hu LL, et al. S-nitrosylation of Hsp90 promotes cardiac hypertrophy in mice through GSK3β signaling. Acta Pharmacol Sin. 2022;43:1979–88.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407.
Macdonald MR, Petrie MC, Hawkins NM, Petrie JR, Fisher M, Mckelvie R, et al. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. 2008;29:1224–40.
Wang Y, Zhang M, Wang R, Lin J, Ma Q, Guo H, et al. Therapeutic inhibition of LincRNA-p21 protects against cardiac hypertrophy. Circ Res. 2024;135:434–49.
Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–71.
Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao Y, et al. USP25 ameliorates pathological cardiac hypertrophy by stabilizing SERCA2a in cardiomyocytes. Circ Res. 2023;132:465–80.
Wang Q, Wang Y, West TM, Liu Y, Reddy GR, Barbagallo F, et al. Carvedilol induces biased β1 adrenergic receptor-nitric oxide synthase 3-cyclic guanylyl monophosphate signalling to promote cardiac contractility. Cardiovasc Res. 2021;117:2237–51.
Kahlon T, Carlisle S, Otero Mostacero D, Williams N, Trainor P, Defilippis AP. Angiotensinogen: more than its downstream products: evidence from population studies and novel therapeutics. JACC Heart Fail 2022;10:699–713.
Wang P, Xu S, Xu J, Xin Y, Lu Y, Zhang H, et al. Elevated MCU expression by CaMKIIδB limits pathological cardiac remodeling. Circulation. 2022;145:1067–83.
Cao Y, Xu C, Ye J, He Q, Zhang X, Jia S, et al. Miro2 regulates inter-mitochondrial communication in the heart and protects against TAC-induced cardiac dysfunction. Circ Res. 2019;125:728–43.
Naito AT, Okada S, Minamino T, Iwanaga K, Liu ML, Sumida T, et al. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ Res. 2010;106:1692–702.
Glembotski CC. Breaking down the COP9 Signalsome in the heart: how inactivating a protein ubiquitin ligase increases protein ubiquitylation and protects the heart. Circ Res. 2015;117:914–6.
Xu N, Gulick J, Osinska H, Yu Y, Mclendon PM, Shay-Winkler K, et al. Ube2v1 positively regulates protein aggregation by modulating ubiquitin proteasome system performance partially through K63 ubiquitination. Circ Res. 2020;126:907–22.
Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–78.
Zhao D, Zhong G, Li J, Pan J, Zhao Y, Song H, et al. Targeting E3 ubiquitin ligase WWP1 prevents cardiac hypertrophy through destabilizing DVL2 via inhibition of K27-linked ubiquitination. Circulation. 2021;144:694–711.
Tang X, Liu X, Sha X, Zhang Y, Zu Y, Fan Q, et al. NEDD4-mediated GSNOR degradation aggravates cardiac hypertrophy and dysfunction. Circ Res. 2025;136:422–38.
Fu L, Cui CP, Zhang X, Zhang L. The functions and regulation of Smurfs in cancers. Semin Cancer Biol. 2020;67:102–16.
Ni C, Chen Y, Xu Y, Zhao J, Li Q, Xiao C, et al. Flavin containing monooxygenase 2 prevents cardiac fibrosis via CYP2J3-SMURF2 axis. Circ Res. 2022; 101161circresaha122320538.
Huang Y, Xu Y, Feng S, He P, Sheng B, Ni J. miR-19b enhances osteogenic differentiation of mesenchymal stem cells and promotes fracture healing through the WWP1/Smurf2-mediated KLF5/β-catenin signaling pathway. Exp Mol Med. 2021;53:973–85.
Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99.
Mund T, Lewis MJ, Maslen S, Pelham HR. Peptide and small molecule inhibitors of HECT-type ubiquitin ligases. Proc Natl Acad Sci USA. 2014;111:16736–41.
Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–56.
Chen X, Li Y, Yuan X, Yuan W, Li C, Zeng Y, et al. Methazolamide attenuates the development of diabetic cardiomyopathy by promoting β-catenin degradation in type 1 diabetic mice. Diabetes. 2022;71:795–811.
Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422.
Liu F, Chen J, Li K, Li H, Zhu Y, Zhai Y, et al. Ubiquitination and deubiquitination in cancer: from mechanisms to novel therapeutic approaches. Mol Cancer. 2024;23:148.
Bernassola F, Chillemi G, Melino G. HECT-type E3 ubiquitin ligases in cancer. Trends Biochem Sci. 2019;44:1057–75.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53.
Liu J, Li W, Deng KQ, Tian S, Liu H, Shi H, et al. The E3 ligase TRIM16 is a key suppressor of pathological cardiac hypertrophy. Circ Res. 2022;130:1586–600.
Lorenzana-Carrillo MA, Tejay S, Nanoa J, Huang G, Liu Y, Haromy A, et al. TRIM35 monoubiquitinates H2B in cardiac cells, implications for heart failure. Circ Res. 2024;135:301–13.
Nayakanti SR, Friedrich A, Sarode P, Jafari L, Maroli G, Boehm M, et al. Targeting Wnt-ß-catenin-FOSL signaling ameliorates right ventricular remodeling. Circ Res. 2023;132:1468–85.
Xiang FL, Fang M, Yutzey KE. Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun. 2017;8:712.
Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res. 2010;107:1198–208.
Kim S, Jho EH. The protein stability of Axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2). J Biol Chem. 2010;285:36420–6.
Hou N, Ye B, Li X, Margulies KB, Xu H, Wang X, et al. Transcription factor 7-like 2 mediates canonical Wnt/β-catenin signaling and c-Myc upregulation in heart failure. Circ Heart Fail. 2016;9:e003010.
Pan L, Huang C, Jin X, Wu J, Jin K, Lin J, et al. Cardiac secreted HSP90α exacerbates pressure overload myocardial hypertrophy and heart failure. Redox Biol. 2025;79:103466.
Tseng PC, Kuo CF, Cheng MH, Wan SW, Lin CF, Chang CP, et al. HECT E3 ubiquitin ligase-regulated Txnip degradation facilitates TLR2-mediated inflammation during Group A Streptococcal infection. Front Immunol. 2019;10:2147.
Kim S, Lee W, Cho K. P62 links the autophagy pathway and the ubiquitin-proteasome system in endothelial cells during atherosclerosis. Int J Mol Sci. 2021;22:7791.
Redondo J, Popik B, Casagrande M, Silva MO, Quillfeldt JA, De Oliveira Alvares L, et al. Hippocampal HECT E3 ligase inhibition facilitates consolidation, retrieval, and reconsolidation, and inhibits extinction of contextual fear memory. Neurobiol Learn Mem. 2020;167:107135.
Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–63.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant No. 82121001, 92468301, 82030013, 82241211, 82222009, 82070278, 82470270, 82170404), the Noncommunicable Chronic Diseases-National Science and Technology Major Project of China (grant No. 2023ZD0503100), and the Natural Science Foundation of Jiangsu Province (BK20240129).
Author information
Authors and Affiliations
Contributions
YJ and LPX contribute to conceptualization; YK, YZ and XML contribute to methodology; YK, QYF and SQC implemented software; XT, YK, YZ and QYF validated; YJ, XT, YK, YZ and QYF conducted investigations; YJ, LPX, XT provided resources; YK, XML and LLR contributes to writing original draft; XT reviewed and edited the text; XT and YK contribute to visualization; YJ, LPX, XT contribute to supervision; YJ, LPX, XT contribute to funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, Y., Zu, Y., Fan, Qy. et al. SMURF2 inhibition attenuates cardiac hypertrophy through blocking ubiquitination degradation of AXIN1. Acta Pharmacol Sin 46, 2951–2962 (2025). https://doi.org/10.1038/s41401-025-01597-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01597-5