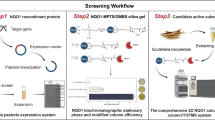
Abstract
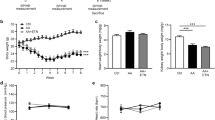
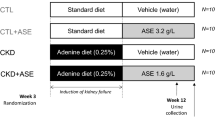
Chronic kidney disease (CKD) is a common disorder with increasing prevalence and morbidity worldwide. However, the present agents do not effectively inhibit pathological processes. Aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that mediated various diseases. Acteoside (ATS) is a commercial ATP-competitive inhibitor of protein kinase C. Clinical study showed ATS mitigated Th22 cell disorder and proteinuria in patients with immunoglobulin A nephropathy. This study analyzed AHR and nuclear factor kappa B (NF-κB) p65 expression in CKD patients. We compared the effects of ATS and its isomer isoacteoside (IAT) on renal function to identify their active functional group of antifibrosis in adenine-induced CKD rats. We further determined the effects of their active functional group on AHR signalling and inflammation pathway. The results showed increasing intrarenal AHR and NF-κB p65 expression in CKD patients. ATS improved renal function and fibrosis while IAT did not significantly improve fibrosis in CKD rats. Both ATS and IAT inhibited intrarenal mRNA expression of AHR and its downstream genes while ATS not IAT significantly inhibited nuclear AHR protein expression. Structure-activity analysis indicated that ATS-containing caffeic acid group by ester bond binding is transformed from C-11 to C-15 becoming IAT leads to a weakened inhibition of fibrosis and AHR nuclear translocation, indicating that caffeic acid group is bioactive functional group of ATS. Furthermore, ATS not IAT significantly regulated protein expression of NF-κB p65 and nuclear factor erythroid 2-related factor 2 (Nrf2) as well as their downstream gene products. Similar results were observed in indole-3-acetic acid (IAA)-induced NRK-52E cells. However, IAT did not show a significant effect. The inhibitory effects of ATS on NF-κB and Nrf2 pathways were partially abolished in IAA-stimulated NRK-52E cells treated with CH223191. However, ATS did not affect AHR expression in IAA-induced NRK-52E cells treated with BAY 11-7082. Therefore, ATS was identified as an AHR antagonist that ameliorated CKD by improving NF-κB/Nrf2 signalling axis. Conclusively, ATS holds promise as a chemical scaffold for the development of new antifibrotic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Francis A, Harhay MN, Ong ACM, Tummalapalli SL, Ortiz A, Fogo AB, et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol. 2024;20:473–85.
Khandpur S, Mishra P, Mishra S, Tiwari S. Challenges in predictive modelling of chronic kidney disease: a narrative review. World J Nephrol. 2024;13:97214.
Balakumar P. Unleashing the pathological role of epithelial-to-mesenchymal transition in diabetic nephropathy: the intricate connection with multifaceted mechanism. World J Nephrol. 2024;13:95410.
Wang YN, Miao H, Yu XY, Guo Y, Su W, Liu F, et al. Oxidative stress and inflammation are mediated via aryl hydrocarbon receptor signalling in idiopathic membranous nephropathy. Free Radic Biol Med. 2023;207:89–106.
Liu D, Chen X, He W, Lu M, Li Q, Zhang S, et al. Update on the pathogenesis, diagnosis, and treatment of diabetic tubulopathy. Integr Med Nephrol Androl. 2024;11:e23–00029.
Miao H, Liu F, Wang YN, Yu XY, Zhuang S, Guo Y, et al. Targeting Lactobacillus johnsonii to reverse chronic kidney disease. Signal Transduct Target Ther. 2024;9:195.
Wang YN, Zhang ZH, Liu HJ, Guo ZY, Zou L, Zhang YM, et al. Integrative phosphatidylcholine metabolism through phospholipase A2 in rats with chronic kidney disease. Acta Pharmacol Sin. 2023;44:393–405.
Correa-Rotter R, Maple-Brown LJ, Sahay R, Tuttle KR. Ulasi II. New and emerging therapies for diabetic kidney disease. Nat Rev Nephrol. 2024;20:156–60.
Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–97.
Miao H, Cao G, Wu XQ, Chen YY, Chen DQ, Chen L, et al. Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177:3415–35.
Cao G, Miao H, Wang YN, Chen DQ, Wu XQ, Chen L, et al. Intrarenal 1-methoxypyrene, an aryl hydrocarbon receptor agonist, mediates progressive tubulointerstitial fibrosis in mice. Acta Pharmacol Sin. 2022;43:2929–45.
Miao H, Wang YN, Yu XY, Zou L, Guo Y, Su W, et al. Lactobacillus species ameliorate membranous nephropathy through inhibiting aryl hydrocarbon receptor pathway via tryptophan-produced indole metabolites. Br J Pharmacol. 2024;181:162–79.
Miao H, Wu XQ, Wang YN, Chen DQ, Chen L, Vaziri ND, et al. 1-Hydroxypyrene mediates renal fibrosis through aryl hydrocarbon receptor signalling pathway. Br J Pharmacol. 2022;179:103–24.
Hu Q, Jiang L, Yan Q, Zeng J, Ma X, Zhao Y. A natural products solution to diabetic nephropathy therapy. Pharmacol Ther. 2023;241:108314.
Guo ZY, Wu X, Zhang SJ, Yang JH, Miao H, Zhao YY. Poria cocos: traditional uses, triterpenoid components and their renoprotective pharmacology. Acta Pharmacol Sin. 2024;46:836–51.
Ruan Z, Liu J, Liu W, Huang W. Qufeng tongluo decoction may alleviate podocyte injury induced by high glucose and hydrogen peroxide by regulating autophagy. Integr Med Nephrol Androl. 2024;11:e24–00023.
Zou TF, Liu ZG, Cao PC, Zheng SH, Guo WT, Wang TX, et al. Fisetin treatment alleviates kidney injury in mice with diabetes-exacerbated atherosclerosis through inhibiting CD36/fibrosis pathway. Acta Pharmacol Sin. 2023;44:2065–74.
Xiao Y, Ren Q, Wu L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed Pharmacother. 2022;153:113296.
Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–12.
Herbert JM, Maffrand JP, Taoubi K, Augereau JM, Fouraste I, Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J Nat Prod. 1991;54:1595–600.
Cheimonidi C, Samara P, Polychronopoulos P, Tsakiri EN, Nikou T, Myrianthopoulos V, et al. Selective cytotoxicity of the herbal substance acteoside against tumor cells and its mechanistic insights. Redox Biol. 2018;16:169–78.
Hsieh PF, Yu CC, Chu PM, Hsieh PL. Verbascoside protects gingival cells against high glucose-induced oxidative stress via PKC/HMGB1/RAGE/NFκB pathway. Antioxidants (Basel). 2021;10:1445.
Seo ES, Oh BK, Pak JH, Yim SH, Gurunathan S, Kim YP, et al. Acteoside improves survival in cecal ligation and puncture-induced septic mice via blocking of high mobility group box 1 release. Mol Cells. 2013;35:348–54.
Wang HQ, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21:368–78.
Zhang Y, Yuan Y, Wu H, Xie Z, Wu Y, Song X, et al. Effect of verbascoside on apoptosis and metastasis in human oral squamous cell carcinoma. Int J Cancer. 2018;143:980–91.
Gan L, Li X, Zhu M, Chen C, Luo H, Zhou Q. Acteoside relieves mesangial cell injury by regulating Th22 cell chemotaxis and proliferation in IgA nephropathy. Ren Fail. 2018;40:364–70.
Mao Y, Yu J, Da J, Yu F, Zha Y. Acteoside alleviates UUO-induced inflammation and fibrosis by regulating the HMGN1/TLR4/TREM1 signaling pathway. PeerJ. 2023;11:e14765.
Gao W, Zhou Y, Li C, Liu T, Zhao H, Wang M, et al. Studies on the metabolism and mechanism of acteoside in treating chronic glomerulonephritis. J Ethnopharmacol. 2023;302:115866.
Wang Q, Dai X, Xiang X, Xu Z, Su S, Wei D, et al. A natural product of acteoside ameliorate kidney injury in diabetes db/db mice and HK-2 cells via regulating NADPH/oxidase-TGF-β/Smad signaling pathway. Phytother Res. 2021;35:5227–40.
Bai YH, Yang M, Feng ZJ, Zheng S. Acteoside ameliorates diabetic kidney disease via regulating the activation of the PPARγ/β-catenin pathway. ScienceAsia. 2021;47:293–302.
Choi CG, Lee DJ, Chung N, Joo YH. Anti-obesity effects of isoacteoside on 3T3-L1 adipocytes. Appl Biol Chem. 2022;65:33.
Wang B, Li XH, Song Z, Li ML, Wu XW, Guo MX, et al. Isoacteoside attenuates acute kidney injury induced by severe acute pancreatitis. Mol Med Rep. 2021;23:287.
Wang YN, Miao H, Hua MR, Yang JZ, Pei M, Yu HX, et al. Moshen granule ameliorates membranous nephropathy by blocking intrarenal renin-angiotensin system signalling via the Wnt1/β-catenin pathway. Phytomedicine. 2023;114:154763.
Miao H, Wang YN, Su W, Zou L, Zhuang SG, Yu XY, et al. Sirtuin 6 protects against podocyte injury by blocking the renin-angiotensin system by inhibiting the Wnt1/β-catenin pathway. Acta Pharmacol Sin. 2023;45:137–49.
Kishi S, Nagasu H, Kidokoro K, Kashihara N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat Rev Nephrol. 2024;20:101–19.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–52.
Rashid I, Tiwari P, D’Cruz S, Jaswal S. Prognostic importance of neutrophil-lymphocyte ratio in non-dialysis chronic kidney disease patients—a hospital-based prospective cohort. Explor Med. 2023;4:299–313.
Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802.
Ndongo M, Nehemie LM, Coundoul B, Diouara AAM, Seck SM. Prevalence and outcomes of polycystic kidney disease in African populations: a systematic review. World J Nephrol. 2024;13:90402.
Mullowney MW, Duncan KR, Elsayed SS, Garg N, van der Hooft JJJ, Martin NI, et al. Artificial intelligence for natural product drug discovery. Nat Rev Drug Discov. 2023;22:895–916.
Shao YF, Tang BB, Ding YH, Fang CY, Hong L, Shao CX, et al. Kaempferide ameliorates cisplatin-induced nephrotoxicity via inhibiting oxidative stress and inducing autophagy. Acta Pharmacol Sin. 2023;44:1442–54.
Zhong X, Jia J, Tan R, Wang L. Hederagenin improves adriamycin-induced nephropathy by inhibiting the JAK/STAT signaling pathway. Integr Med Nephrol Androl. 2024;11:e22–00016.
Wang B, Yang LN, Yang LT, Liang Y, Guo F, Fu P, et al. Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis. Acta Pharmacol Sin. 2024;45:150–65.
Vagopoulou A, Theofilis P, Karasavvidou D, Haddad N, Makridis D, Tzimikas S, et al. Pilot study on the effect of flavonoids on arterial stiffness and oxidative stress in chronic kidney disease. World J Nephrol. 2024;13:95262.
Du W, Di Martino L, Li J. Natural polysaccharides-based postbiotics and their potential applications. Explor Med. 2024;5:444–58.
Uddin MJ, Çiçek SS, Willer J, Shulha O, Abdalla MA, Sönnichsen F, et al. Phenylpropanoid and flavonoid glycosides from the leaves of Clerodendrum infortunatum (Lamiaceae). Biochem Syst Ecol. 2020;92:104131.
Jiang X, Wang J, Lin L, Du L, Ding Y, Zheng F, et al. Macrophages promote pre-metastatic niche formation of breast cancer through aryl hydrocarbon receptor activity. Signal Transduct Target Ther. 2024;9:352.
Sládeková L, Mani S, Dvořák Z. Ligands and agonists of the aryl hydrocarbon receptor AhR: Facts and myths. Biochem Pharmacol. 2023;213:115626.
Salminen A. Aryl hydrocarbon receptor (AhR) impairs circadian regulation: Impact on the aging process. Ageing Res Rev. 2023;87:101928.
Pinto CJG, Ávila-Gálvez M, Lian Y, Moura-Alves P, Nunes Dos Santos C. Targeting the aryl hydrocarbon receptor by gut phenolic metabolites: a strategy towards gut inflammation. Redox Biol. 2023;61:102622.
Vogel CFA. Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530.
Dong F, Perdew GH. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. 2020;12:1859812.
Zhao H, Chen L, Yang T, Feng YL, Vaziri ND, Liu BL, et al. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J Transl Med. 2019;17:302.
Ying Y, Song LY, Pang WL, Zhang SQ, Yu JZ, Liang PT, et al. Astragalus polysaccharide protects experimental colitis through an aryl hydrocarbon receptor-dependent autophagy mechanism. Br J Pharmacol. 2024;181:681–97.
Mo Y, Hu D, Yu W, Ji C, Li Y, Liu X, et al. Astragaloside IV attenuates indoxyl sulfate-induced injury of renal tubular epithelial cells by inhibiting the aryl hydrocarbon receptor pathway. J Ethnopharmacol. 2023;308:116244.
Li MZ, Zhao Y, Wang HR, Talukder M, Li JL. Lycopene preventing DEHP-induced renal cell damage is targeted by aryl hydrocarbon receptor. J Agric Food Chem. 2021;69:12853–61.
Liu X, Deng R, Chen Y, Huang S, Lu J, Zheng L, et al. Jian-Pi-Yi-Shen formula improves adenine-induced chronic kidney disease via regulating tryptophan metabolism and aryl hydrocarbon receptor signaling. Front Pharmacol. 2022;13:922707.
Mo Y, Jie X, Wang L, Ji C, Gu Y, Lu Z, et al. Bupi Yishen formula attenuates kidney injury in 5/6 nephrectomized rats via the tryptophan-kynurenic acid-aryl hydrocarbon receptor pathway. BMC Complement Med Ther. 2021;21:207.
Ren N, Wang WF, Zou L, Zhao YL, Miao H, Zhao YY. The nuclear factor kappa B signaling pathway is a master regulator of renal fibrosis. Front Pharmacol. 2023;14:1335094.
Wang YN, Li XJ, Wang WF, Zou L, Miao H, Zhao YY. Geniposidic acid attenuates chronic tubulointerstitial nephropathy through regulation of the NF‐κB/Nrf2 pathway via aryl hydrocarbon receptor signaling. Phytother Res. 2024;38:5441–57.
Li XJ, Wang YN, Wang WF, Nie X, Miao H, Zhao YY. Barleriside A, an aryl hydrocarbon receptor antagonist, ameliorates podocyte injury through inhibiting oxidative stress and inflammation. Front Pharmacol. 2024;15:1386604.
Naim MJ. A review on mushrooms as a versatile therapeutic agent with emphasis on its bioactive constituents for anticancer and antioxidant potential. Explor Med. 2024;5:312–30.
Li XJ, Suo P, Wang YN, Zou L, Nie XL, Zhao YY, et al. Arachidonic acid metabolism as a therapeutic target in AKI-to-CKD transition. Front Pharmacol. 2024;15:1365802.
Jiang YC, Lin X, Mao Y, Zhao JQ, Zhang GH, Yu JL, et al. Acteoside alleviates renal fibrosis by inhibiting β-catenin/CTGF signaling pathway in UUO rats. Nat Prod Commun. 2022;17:1934578X2211348.
Xie Y, Lin X, Yuan J, Dong R, Yu JL, Zha Y. Effects of acteoside on the expressions of MCP-1 and TGF-β1 in the diabetic nephropathy mice. Eur J Inflamm. 2022;20:1721727X221118348.
Zhang X, He H, Liang D, Jiang Y, Liang W, Chi ZH, et al. Protective effects of berberine on renal injury in streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci. 2016;17:1327.
Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401.
Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82.
Zhao H, Zhao T, Li P. Gut microbiota-derived metabolites: a new perspective of traditional Chinese medicine against diabetic kidney disease. Integr Med Nephrol Androl. 2024;11:e23–00024.
Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20.
Li XJ, Shan QY, Wu X, Miao H, Zhao YY. Gut microbiota regulates oxidative stress and inflammation: a double-edged sword in renal fibrosis. Cell Mol Life Sci. 2024;81:480.
Cernaro V, Calabrese V, Loddo S, Corsaro R, Macaione V, Ferlazzo VT, et al. Indole-3-acetic acid correlates with monocyte-to-high-density lipoprotein (HDL) ratio (MHR) in chronic kidney disease patients. Int Urol Nephrol. 2022;54:2355–64.
Nayak S, Boopathi S, Chandrasekar M, Panda SP, Manikandan K, Chitra V, et al. Indole-3-acetic acid exposure leads to cardiovascular inflammation and fibrosis in chronic kidney disease rat model. Food Chem Toxicol. 2024;192:114917.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (82074002, 82274079, 82274192, 82474062), Shaanxi Key Science and Technology Plan Project (2023-ZDLSF-26) and Shaanxi Natural Science Basic Research Program (2025JC-YBQN-1210).
Author information
Authors and Affiliations
Contributions
HM and YYZ conceived and designed the experiments. YNW, XW, QYS and QY conducted and analysed the experiments. HM and YYZ performed statistical analysis. YYZ wrote the initial draft of the manuscript. XYY, JHY and GC revised the manuscript. All authors have critically revised the manuscript and approved its final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Yn., Wu, X., Shan, Qy. et al. Acteoside-containing caffeic acid is bioactive functional group of antifibrotic effect by suppressing inflammation via inhibiting AHR nuclear translocation in chronic kidney disease. Acta Pharmacol Sin 46, 2975–2988 (2025). https://doi.org/10.1038/s41401-025-01598-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01598-4